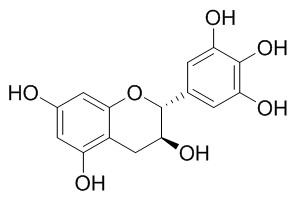
(+)-Gallocatechin
(+)-Gallocatechin as a bio-antimutagenic compound against UV-induced mutation in Escherichia coli. It is potent in scavenging Fremy’s salt, a synthetic free radical, it possesses antioxidant capacities that is higher or comparable to that of ascorbic acid or Trolox.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2024, 29(5):1171.
Asian Pac J Cancer Prev.2021, 22(S1):97-106.
Oxid Med Cell Longev.2021, 2021:6647107.
J Pharm Biomed Anal.2024, 241:115990.
Antibiotics.2022, 11(4), 510.
Evid Based Complement Alternat Med.2019, 2019:2135351
Pharmaceuticals (Basel).2020, 13(9):262.
Heliyon2020, 6(6):e04337.
Molecules.2024, 29(5):1050.
Molecules 2022, 27(3),1047.
Related and Featured Products
Phytochemistry. 1994 Jul;36(4):1027-9.
Identification of (+)-gallocatechin as a bio-antimutagenic compound in Psidium guava leaves.[Pubmed:
7765204]
From the MeOH-extract of guava leaves, (+)-Gallocatechin was isolated as a bio-antimutagenic compound against UV-induced mutation in Escherichia coli. This strengthens the evidence that phenolic compounds require three neighbouring-OH groups in order to possess this activity.
Eur Food Res. Technol., 2004, 219(6):605-13.
Antioxidant gallocatechins, dimeric and trimeric proanthocyanidins from sea buckthorn (Hippophaë rhamnoides) pomace[Reference:
WebLink]
Residues such as peels and seeds that result from fruit juice production may contain substantial amounts of valuable natural antioxidants.
METHODS AND RESULTS:
In order to isolate the potential antioxidants gallocatechins and proanthocyanidins, pomace from sea buckthorn (Hippophaë rhamnoides) berries was extracted by acetone-water (75:25, v/v) and fractionated by Sephadex LH-20 gel chromatography and semipreparative HPLC. The structures of the monomeric flavan-3-ols isolated, (+)-Gallocatechin and (−)-epigallocatechin, were elucidated by electrospray mass spectrometry (ESI-MS) and 1H and 13C NMR spectroscopy. Five dimeric proanthocyanidins were identified by HPLC-ESI-MS(-MS) and by acid-catalyzed cleavage in the presence of phloroglucinol. Moreover, nine trimeric proanthocyanidins were tentatively identified by HPLC-ESI-MS(-MS) in the Sephadex fractions of sea buckthorn pomace. The isolated flavan-3-ols and proanthocyanidins were potent in scavenging Fremy’s salt, a synthetic free radical.
CONCLUSIONS:
They possessed antioxidant capacities that were higher or comparable to that of ascorbic acid or Trolox. On comparing the antioxidant capacities of monomeric flavan-3-ols and dimeric proanthocyanidins, no significant influence from the degree of polymerization (DP) was observed.