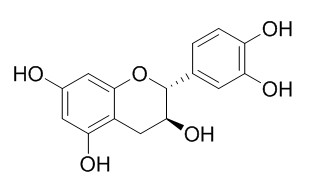
Catechin
Catechin, a cyclooxygenase-1 (COX-1) inhibitor with an IC50 of 1.4 μM, which has antiangiogenic, antitumor, antioxidant, UV-protective, anti-aging, phytotoxic, antimicrobial, and antiviral effects. Catechin shows its potential as biobased active packaging for fatty food, and exerts cardioprotection through treating many kinds of angiocardiopathy.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Microchemical Journal2018, 137:168-173
Evid Based Complement Alternat Med.2018, 2018:3610494
Acta Physiologiae Plantarum2016, 38:7
Methods Protoc.2024, 7(6):95.
BMC Complement Altern Med.2014, 14:352
Applied Biological Chemistry 2021, 64(75)
Cell.2022, 185(23):4298-4316.e21.
Applied Food Research2024, 100662.
J Korean Soc Food Sci Nutr2020, doi: 10.3746.
Crystals2020, 10(3), 206.
Related and Featured Products
Pharm Dev Technol. 2014 Jun;19(4):395-400.
Effect of emulsification on the skin permeation and UV protection of catechin.[Pubmed:
23639253]
An anti-aging effect may be obtained by skin application of tea Catechins (Camellia sinensis) since they have high ultraviolet (UV)-protection activity.
METHODS AND RESULTS:
In this study, the skin permeation of Catechin (C), epiCatechin (EC), epigalloCatechin (EGC), epiCatechin gallate (ECg) and epigalloCatechin gallate (EGCg) was determined and compared, and the effect of emulsification on the skin permeation of C was measured. The UV-protective effect of C was also determined. The in vitro skin permeability of each Catechin derivative was determined using side-by-side diffusion of cells. The UV-protective effect of C was determined by applying different concentrations of C to the solution or emulsion on a three-dimensional cultured human skin model or normal human epidermal keratinocytes with UV-irradiation. ECg and EGCg with gallate groups showed lower skin permeability than C, EC and EGC without gallate groups, suggesting that the skin permeability of Catechin derivatives may be dependent on the existence of a gallate group. Interestingly, the skin permeation of C was increased by an o/w emulsification. In addition, the C emulsion showed a significantly higher UV-protective effect by C than that with its aqueous solution.
CONCLUSIONS:
These results suggest that the o/w emulsion of Catechin derivatives is probably useful as a cosmetic formulation with anti-aging efficacy.
Biochem Pharmacol. 2011 Dec 15;82(12):1807-21.
Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications.[Pubmed:
21827739 ]
An expanding body of preclinical evidence suggests EGCG, the major Catechin found in green tea (Camellia sinensis), has the potential to impact a variety of human diseases.
METHODS AND RESULTS:
Apparently, EGCG functions as a powerful antioxidant, preventing oxidative damage in healthy cells, but also as an antiangiogenic and antitumor agent and as a modulator of tumor cell response to chemotherapy. Much of the cancer chemopreventive properties of green tea are mediated by EGCG that induces apoptosis and promotes cell growth arrest by altering the expression of cell cycle regulatory proteins, activating killer caspases, and suppressing oncogenic transcription factors and pluripotency maintain factors. In vitro studies have demonstrated that EGCG blocks carcinogenesis by affecting a wide array of signal transduction pathways including JAK/STAT, MAPK, PI3K/AKT, Wnt and Notch. EGCG stimulates telomere fragmentation through inhibiting telomerase activity. Various clinical studies have revealed that treatment by EGCG inhibits tumor incidence and multiplicity in different organ sites such as liver, stomach, skin, lung, mammary gland and colon. Recent work demonstrated that EGCG reduced DNMTs, proteases, and DHFR activities, which would affect transcription of TSGs and protein synthesis. EGCG has great potential in cancer prevention because of its safety, low cost and bioavailability. In this review, we discuss its cancer preventive properties and its mechanism of action at numerous points regulating cancer cell growth, survival, angiogenesis and metastasis.
CONCLUSIONS:
Therefore, non-toxic natural agent could be useful either alone or in combination with conventional therapeutics for the prevention of tumor progression and/or treatment of human malignancies.
Cytotechnology . 2018 Feb;70(1):245-259.
Catechin ameliorates doxorubicin-induced neuronal cytotoxicity in in vitro and episodic memory deficit in in vivo in Wistar rats[Pubmed:
28900743]
Cognitive dysfunction by chemotherapy compromises the quality of life in cancer patients. Tea polyphenols are known chemopreventive agents. The present study was designed to evaluate the neuroprotective potential of (+) Catechin hydrate (Catechin), a tea polyphenol, in IMR-32 neuroblastoma cells in vitro and alleviation of episodic memory deficit in Wistar rats in vivo against a widely used chemotherapeutic agent, Doxorubicin (DOX). In vitro, neuroprotective studies were assessed in undifferentiated IMR-32 cells using percentage viability and in differentiated cells by neurite length. These studies showed Catechin increased percentage viability of undifferentiated IMR-32 cells. Catechin pretreatment also showed an increase in neurite length of differentiated cells. In vivo neuroprotection of Catechin was evaluated using novel object recognition task in time-induced memory deficit model at 50, 100 and 200 mg/kg dose and DOX-induced memory deficit models at 100 mg/kg dose. The latter model was developed by injection of DOX (2.5 mg/kg, i.p.) in 10 cycles over 50 days in Wistar rats. Catechin showed a significant reversal of time-induced memory deficit in a dose-dependent manner and prevention of DOX-induced memory deficit at 100 mg/kg. In addition, Catechin treatment showed a significant decrease in oxidative stress, acetylcholine esterase and neuroinflammation in the hippocampus and cerebral cortex in DOX-induced toxicity model. Hence, Catechin may be a potential adjuvant therapy for the amelioration of DOX-induced cognitive impairment which may improve the quality of life of cancer survivors. This improvement might be due to the elevation of antioxidant defense, prevention of neuroinflammation and inhibition of acetylcholine esterase enzyme.
Am J Clin Nutr. 2001 Aug;74(2):227-32.
Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study.[Pubmed:
11470725]
Epidemiologic studies suggest that tea consumption may reduce the risk of cardiovascular diseases, but results are inconsistent. Catechins, which belong to the flavonoid family, are the main components of tea and may be responsible for the alleged protective effect. Taking Catechin sources other than tea into account might clarify the reported associations.
METHODS AND RESULTS:
The objective was to evaluate the association between Catechin intake and the incidence of and mortality from ischemic heart disease and stroke.
We evaluated the effect of a high Catechin intake by using data from the Zutphen Elderly Study, a prospective cohort study of 806 men aged 65-84 y at baseline in 1985.
The mean (+/-SD) Catechin intake at baseline was 72 +/- 47.8 mg, mainly from black tea, apples, and chocolate. A total of 90 deaths from ischemic heart disease were documented. Catechin intake was inversely associated with ischemic heart disease mortality; the multivariate-adjusted risk ratio in the highest tertile of intake was 0.49 (95% CI: 0.27, 0.88; P for trend: 0.017). After multivariate adjustment, Catechin intake was not associated with the incidence of myocardial infarction (risk ratio in the highest tertile of intake: 0.70; 95% CI: 0.39, 1.26; P for trend: 0.232). After adjustment for tea consumption and flavonol intake, a 7.5-mg increase in Catechin intake from sources other than tea was associated with a tendency for a 20% reduction in ischemic heart disease mortality risk (P = 0.114). There was no association between Catechin intake and stroke incidence or mortality.
CONCLUSIONS:
Catechins, whether from tea or other sources, may reduce the risk of ischemic heart disease mortality but not of stroke.
Antiviral Res. 2005 Nov;68(2):66-74.
Antiviral effect of catechins in green tea on influenza virus.[Pubmed:
16137775 ]
Polyphenolic compound Catechins ((-)-epigalloCatechin gallate (EGCG), (-)-epiCatechin gallate (ECG) and (-)-epigalloCatechin (EGC)) from green tea were evaluated for their ability to inhibit influenza virus replication in cell culture and for potentially direct virucidal effect.
METHODS AND RESULTS:
Among the test compounds, the EGCG and ECG were found to be potent inhibitors of influenza virus replication in MDCK cell culture and this effect was observed in all influenza virus subtypes tested, including A/H1N1, A/H3N2 and B virus. The 50% effective inhibition concentration (EC50) of EGCG, ECG, and EGC for influenza A virus were 22-28, 22-40 and 309-318 microM, respectively. EGCG and ECG exhibited hemagglutination inhibition activity, EGCG being more effective. However, the sensitivity in hemagglutination inhibition was widely different among three different subtypes of influenza viruses tested. Quantitative RT-PCR analysis revealed that, at high concentration, EGCG and ECG also suppressed viral RNA synthesis in MDCK cells whereas EGC failed to show similar effect. Similarly, EGCG and ECG inhibited the neuraminidase activity more effectively than the EGC. The results show that the 3-galloyl group of Catechin skeleton plays an important role on the observed antiviral activity, whereas the 5'-OH at the trihydroxy benzyl moiety at 2-position plays a minor role.
CONCLUSIONS:
The results, along with the HA type-specific effect, suggest that the antiviral effect of Catechins on influenza virus is mediated not only by specific interaction with HA, but altering the physical properties of viral membrane.
Inflamm Res. 2014 Aug;63(8):619-28.
Catechin ameliorates cardiac dysfunction in rats with chronic heart failure by regulating the balance between Th17 and Treg cells.[Pubmed:
24760105]
Disequilibrium of the cytokine network was reported to play an important role in the progression of chronic heart failure (CHF). Catechin exerts cardioprotection through treating many kinds of angiocardiopathy. However, the effects of Catechin on CHF are currently unclear. Therefore, the main aim of this study was to investigate the efficacy of Catechin on CHF rats as well as its relationship to immunoregulation.
METHODS AND RESULTS:
CHF was induced in rats by ligation of the abdominal aorta. Myocardial function was evaluated by left ventricular systolic pressure and left ventricular end-diastolic pressure. The cytokine level was measured by enzyme-linked immunosorbent assay. Th17 and Treg levels in peripheral blood and spleen were analyzed by flow cytometry.
The results showed that Catechin treatment (50, 100 mg/kg/day) markedly improved myocardial function in rats treated with abdominal aortic coarctation. Severity of myocardial dysfunction in CHF rats significantly correlated with serum values of interleukin-17 (IL-17)/IL-10. Further results indicated Catechin obviously inhibited immune activation, regulated unbalanced levels of IL-17/IL-10, and reversed abnormal polarization of TH17 as well as Treg in peripheral blood and spleen.
CONCLUSIONS:
Taken together, oral administration of Catechin effectively suppressed abdominal aorta ligation-induced CHF in rats, which was closely associated with its modulation on Th17 and Treg.
J Agric Food Chem. 2014 Oct 15;62(41):10170-80.
Plasticized poly(lactic acid)-poly(hydroxybutyrate) (PLA-PHB) blends incorporated with catechin intended for active food-packaging applications.[Pubmed:
25255375]
METHODS AND RESULTS:
Active biobased packaging materials based on poly(lactic acid)-poly(hydroxybutyrate) (PLA-PHB) blends were prepared by melt blending and fully characterized. Catechin incorporation, as antioxidant compound, enhanced the thermal stability, whereas its release was improved by the addition of acetyl(tributyl citrate) (ATBC) as plasticizer. Whereas the incorporation of ATBC resulted in a reduction of elastic modulus and hardness, Catechin addition produced more rigid materials due to hydrogen-bonding interactions between Catechin hydroxyl groups and carbonyl groups of PLA and PHB. The quantification of Catechin released into a fatty food simulant and the antioxidant effectiveness after the release process were demonstrated. The effect of the materials' exposure to a food simulant was also investigated. PHB-added materials maintained their structural and mechanical properties after 10 days in a test medium that represents the worst foreseeable conditions of the intended use.
CONCLUSIONS:
Thus, plasticized PLA-PHB blends with Catechin show their potential as biobased active packaging for fatty food.
Bioorg Med Chem Lett. 2014 Jun 1;24(11):2582-4.
Synthesis and radical-scavenging activity of a dimethyl catechin analogue.[Pubmed:
24792463]
Catechin analogue 1 with methyl substituents ortho to the catechol hydroxyl groups was synthesized to improve the antioxidant ability of (+)-Catechin. The synthetic scheme involved a solid acid catalyzed Friedel-Crafts coupling of a cinnamyl alcohol derivative to 3,5-dibenzyloxyphenol followed by hydroxylation and then cyclization through an intermediate orthoester.
The antioxidative radical scavenging activity of 1 against galvinoxyl radical, an oxyl radical, was found to be 28-fold more potent than (+)-Catechin.