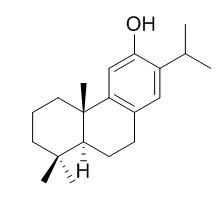
Ferruginol
Ferruginol has anti-plasmodial, leishmanicidal, anti-ulcerogenic, cardioprotective, anticancer, anti-oxidative and anti-inflammatory activities, it can induce apoptosis in non-small cell lung cancer (NSCLC) cells. Ferruginol has antimicrobial and antifungal activities against wood-rot fungi (basidiomycetes), can cause cellular dysfunction and damage, lead to growth inhibition and autophagic cell death of fungi.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J of App. Res. on Med&Aromatic Plants2020, 100291.
Journal of Life Science2017, 233-240
Antioxidants (Basel).2022, 11(8):1471.
ACS Chem Biol.2019, 14(5):873-881
Planta Med.2018, 84(6-07):465-474
Heliyon.2023, 9(11):e21944.
Antioxidants (Basel).2020, 9(4):326.
Toxins (Basel).2023, 15(3):231.
Food Hydrocolloids2024, 57:110432
Pol J Microbiol.2021, 70(1):117-130.
Related and Featured Products
Ann Bot. 2014 May;113(6):1029-36.
The accumulation pattern of ferruginol in the heartwood-forming Cryptomeria japonica xylem as determined by time-of-flight secondary ion mass spectrometry and quantity analysis.[Pubmed:
24651372]
Heartwood formation is a unique phenomenon of tree species. Although the accumulation of heartwood substances is a well-known feature of the process, the accumulation mechanism remains unclear. The aim of this study was to determine the accumulation process of Ferruginol, a predominant heartwood substance of Cryptomeria japonica, in heartwood-forming xylem.
METHODS AND RESULTS:
The radial accumulation pattern of Ferruginol was examined from sapwood and through the intermediate wood to the heartwood by direct mapping using time-of-flight secondary ion mass spectrometry (TOF-SIMS). The data were compared with quantitative results obtained from a novel method of gas chromatography analysis using laser microdissection sampling and with water distribution obtained from cryo-scanning electron microscopy. Ferruginol initially accumulated in the middle of the intermediate wood, in the earlywood near the annual ring boundary. It accumulated throughout the entire earlywood in the inner intermediate wood, and in both the earlywood and the latewood in the heartwood. The process of Ferruginol accumulation continued for more than eight annual rings. Ferruginol concentration peaked at the border between the intermediate wood and heartwood, while the concentration was less in the latewood compared with the earlywood in each annual ring. Ferruginol tended to accumulate around the ray parenchyma cells. In addition, at the border between the intermediate wood and heartwood, the accumulation was higher in areas without water than in areas with water.
CONCLUSIONS:
TOF-SIMS clearly revealed Ferruginol distribution at the cellular level. Ferruginol accumulation begins in the middle of intermediate wood, initially in the earlywood near the annual ring boundary, then throughout the entire earlywood, and finally across to the whole annual ring in the heartwood. The heterogeneous timing of Ferruginol accumulation could be related to the distribution of ray parenchyma cells and/or water in the heartwood-forming xylem.
J Agric Food Chem. 2015 Jan 14;63(1):85-91.
Proteomics investigation reveals cell death-associated proteins of basidiomycete fungus Trametes versicolor treated with Ferruginol.[Pubmed:
25485628]
Ferruginol has antifungal activity against wood-rot fungi (basidiomycetes). However, specific research on the antifungal mechanisms of Ferruginol is scarce.
METHODS AND RESULTS:
Two-dimensional gel electrophoresis and fluorescent image analysis were employed to evaluate the differential protein expression of wood-rot fungus Trametes versicolor treated with or without Ferruginol. Results from protein identification of tryptic peptides via liquid chromatography–electrospray ionization tandem mass spectrometry (LC–ESI-MS/MS) analyses revealed 17 protein assignments with differential expression. Downregulation of cytoskeleton β-tubulin 3 indicates that Ferruginol has potential to be used as a microtubule-disrupting agent. Downregulation of major facilitator superfamily (MFS)–multiple drug resistance (MDR) transporter and peroxiredoxin TSA1 were observed, suggesting reduction in self-defensive capabilities of T. versicolor. In addition, the proteins involved in polypeptide sorting and DNA repair were also downregulated, while heat shock proteins and autophagy-related protein 7 were upregulated.
CONCLUSIONS:
These observations reveal that such cellular dysfunction and damage caused by Ferruginol lead to growth inhibition and autophagic cell death of fungi.
Bioorg Med Chem Lett. 2014 Nov 15;24(22):5234-7.
Antimalarial activity of abietane ferruginol analogues possessing a phthalimide group.[Pubmed:
25316317]
The abietane-type diterpenoid (+)-Ferruginol, a bioactive compound isolated from New Zealand's Miro tree (Podocarpus ferruginea), displays relevant pharmacological properties, including antimicrobial, cardioprotective, anti-oxidative, anti-plasmodial, leishmanicidal, anti-ulcerogenic, anti-inflammatory and anticancer.
METHODS AND RESULTS:
Herein, we demonstrate that Ferruginol (1) and some phthalimide containing analogues 2-12 have potential antimalarial activity. The compounds were evaluated against malaria strains 3D7 and K1, and cytotoxicity was measured against a mammalian cell line panel. A promising lead, compound 3, showed potent activity with an EC50 = 86 nM (3D7 strain), 201 nM (K1 strain) and low cytotoxicity in mammalian cells (SI>290). Some structure-activity relationships have been identified for the antimalarial activity in these abietane analogues.
Integr Cancer Ther. 2015 Jan;14(1):86-97.
Ferruginol inhibits non-small cell lung cancer growth by inducing caspase-associated apoptosis.[Pubmed:
25355727]
The anti-lung cancer effect of Cryptomeria japonica leaf extractive and its active phytocompound was evaluated using in vitro and in vivo assays.
METHODS AND RESULTS:
The anti-lung cancer mechanism was investigated using flow cytometry and western blot analyses, and the antitumor activity was evaluated in a xenograft animal model. MTT assay indicated that the cytotoxic effects of Ferruginol in A549 and CL1-5 cells were dose-dependent. According to the results of cell cycle and annexin V/PI analyses, the sub-G1 population and annexin V binding in the 2 cell lines were increased after Ferruginol treatment. The results of western blot analyses revealed that the cleaved forms of caspase 3, 8, 9, and poly(ADP-ribose) polymerase were activated after Ferruginol treatment in A549 and CL1-5 cells. Moreover, the expression of the anti-apoptotic protein Bcl-2 was decreased, while the expression of the pro-apoptotic protein Bax was elevated, after Ferruginol treatment in both lung cancer cell lines. These results indicated that Ferruginol acted via a caspase-dependent mitochondrial apoptotic pathway in the 2 cell lines. Intraperitoneal administration of Ferruginol significantly suppressed the growth of subcutaneous CL1-5 xenografts.
CONCLUSIONS:
The findings of the present study provided insight into the molecular mechanisms underlying Ferruginol-induced apoptosis in non-small cell lung cancer (NSCLC) cells, indicating that this compound may be a potential candidate drug for anti-NSCLC.