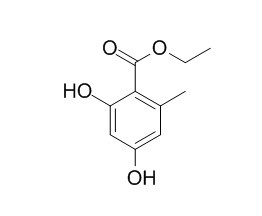
Ethyl orsellinate
Ethyl orsellinate shows good inhibition of protein glycation, and urease activities.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Pharmacognosy Magazine2024, 20(2):632-645.
Pharmacia2022, 69(3): 883-890.
Sci Rep.2018, 8(1)
Korean J. Medicinal Crop Sci.2021, 29(6):425-433
Agronomy2022, 12(10), 2426.
Food Res Int.2018, 106:909-919
Nutrients.2020, 12(5):1242.
Molecules.2019, 24(19):E3417
Evid Based Complement Alternat Med.2021, 2021:8850744.
Biochem Pharmacol. 2020, 177:114014.
Related and Featured Products
Science China Chemistry, 2011 ,54 (12) :1926-1931.
New antiglycation and enzyme inhibitors from Parmotrema cooperi[Reference:
WebLink]
Lichens are unique individuals which have been widely used in traditional medicines. This study was focused on the bioassay-guided phytochemical investigation, and bioactivity evaluation on a lichens species, Parmotrema cooperi.
METHODS AND RESULTS:
This first bioassay-directed chemical study on P. cooperi has led to the isolation of ethyl heamatomate (1), atraric acid (2), Ethyl orsellinate (3), orsellinic acid (4), lecanoric acid (5), gyrophoric acid (6), and licanorin (7). The structures of 1–7 were mainly elucidated from spectroscopic methods including 1D, and 2D NMR spectroscopy, and mass spectrometry. These compounds were evaluated for their antiglycation, urease, α-chymotrypsin, and β-glucoronidase inhibitory activities.
CONCLUSIONS:
Few of the phenolic compounds showed significant, while most of them showed good inhibition of protein glycation, and urease activities.
Zhongguo Zhong Yao Za Zhi. 2011 Aug;36(16):2233-5.
Determination of atranol, lecanorin, ethyl orsellinate and methyl orsellinate in Usnea diffracta by RP-HPLC.[Pubmed:
22097337 ]
To develop a RP-HPLC method for determining the contents of atranol, lecanorin, Ethyl orsellinate and mEthyl orsellinate in Usnea diffracta.
METHODS AND RESULTS:
A Kromasil-C18 column (4.6 mm x 250 mm, 5 microm) was used at 25 degrees C with the mobile phases of acetonitrile -1% acetic acid in a gradient manner. The flow rate was set at 1.0 mL x min(-1). The detection wavelength was 280 nm.
The correlation coefficients of atranol, lecanorin, Ethyl orsellinate, and mEthyl orsellinate were higher than 0.999. Recoveries were from 102.9% to 95.30%; with RSD from 2.3% to 1.9%.
CONCLUSIONS:
The method is quick, simple and repeatable for simultaneous determination of atranol, lecanorin, Ethyl orsellinate and mEthyl orsellinate in U. diffracta.