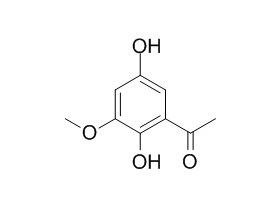
Acetophenone
Acetophenones and chalcones may represent good leads in the discovery of new nematicidal compounds and may have potential use in crop management as active ingredients.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Antioxidants (Basel).2021, 10(8):1300.
ACS Omega.2024, 9(41):42227-42244.
J Med Food.2022, 25(3):272-280.
Journal of Ginseng Research2021, 25 November
J Adv Res.2019, 17:85-94
Bull. Pharm. Sci., Assiut University2020, 43(2):149-155.
Journal of Physiology & Pathology in Korean Medicine.2018, 32(2): 106-112
Genes (Basel).2021, 12(7):1024.
J Holistic Integrative Pharm.2023, 4(1):14-28
Molecules2022, 27(3),1140.
Related and Featured Products
Pest Manag Sci. 2015 Jan 16.
Nematicidal activity of acetophenones and chalcones against Meloidogyne incognita and structure-activity considerations.[Pubmed:
25641877]
With the ultimate goal of identifying new compounds active against root-knot nematodes, a set of 14 substituted chalcones were synthesised, starting from Acetophenones. These chalcones and various Acetophenones were tested in vitro against Meloidogyne incognita.
METHODS AND RESULTS:
The most potent Acetophenones were 4-nitroAcetophenone and 4-iodoAcetophenone, with EC50 /24 h values of 12 ± 5 and 15 ± 4 mg L-1 respectively, somewhat weaker than that of the chemical control fosthiazate in our previous experiments (EC50 /24 h 0.4 ± 0.3 mg L-1 ). When we converted the Acetophenones to chalcones, the nematicidal activity differed, based on their substitution pattern. The condensation of 4-nitroAcetophenone with 2,4,6-trihydroxybenzaldehyde to give the corresponding chalcone (E)-1-(4-nitrophenyl)-3-(2,4,6-trihydroxyphenyl)prop-2-en-1-one led to a slight reduction in activity (EC50 /24 h value 25 ± 17 mg L-1 ). Moreover, (E)-3-(2-hydroxy-5-iodophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one showed better activity (EC50 /24 h value 26 ± 15 mg L-1 ) than 4-methoxyAcetophenone (EC50 /24 h value 43 ± 10 mg L-1 ).
CONCLUSIONS:
Acetophenones and chalcones may represent good leads in the discovery of new nematicidal compounds and may have potential use in crop management as active ingredients. © 2015 Society of Chemical Industry.