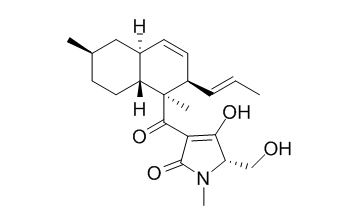
Equisetin
Equisetin has antibacterial activity, the MIC's for Equisetin are 8 ug /mL against Bacillus subtilis, 16 ug /mL against Staphylococcus aureus and Methicillin Resistant Staphylococcus aureus (MRSA).Equisetin inhibits the DNP-stimulated ATPase activity of rat liver mitochondria and mitoplasts in a concentration-dependent manner; 50% inhibition is caused by about 8 nmol Equisetin/mg protein.It also specifically inhibits the substrate anion carriers of the mitochondrial inner membrane.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Sci Rep. 2017, 12953(7)
LWT2020, 124:109163
Curr Eye Res.2018, 43(1):27-34
Hong Kong Baptist University2023, 048330T.
Int J Mol Sci.2019, 20(9):E2244
Research Square2022, rs.3.rs-1948239
J Nat Med.2021, doi: 10.1007.
Phytochemistry.2021, 181:112539.
J Sep Sci.2018, 41(11):2488-2497
Drug Test Anal.2018, 10(10):1579-1589
Related and Featured Products
BMC Complement Altern Med. 2015 Jul 10;15:220.
Antimicrobial activities of endophytic fungi obtained from the arid zone invasive plant Opuntia dillenii and the isolation of equisetin, from endophytic Fusarium sp.[Pubmed:
26160390]
Opuntia dillenii is an invasive plant well established in the harsh South-Eastern arid zone of Sri Lanka. Evidence suggests it is likely that the endophytic fungal populations of O. dillenii assist the host in overcoming biotic and abiotic stress by producing biologically active metabolites. With this in mind there is potential to discover novel natural products with useful biological activities from this hitherto poorly investigated source. Consequently, an investigation of the antimicrobial activities of the endophytes of O. dillenii, that occupies a unique ecological niche, may well provide useful leads in the discovery of new pharmaceuticals.
METHODS AND RESULTS:
Endophytic fungi were isolated from the surface sterilized cladodes and flowers of O. dillenii using several nutrient media and the antimicrobial activities were evaluated against three Gram-positive and two Gram-negative bacteria and Candida albicans. The two most bioactive fungi were identified by colony morphology and DNA sequencing. The secondary metabolite of the endophyte Fusarium sp. exhibiting the best activity was isolated via bioassay guided chromatography. The chemical structure was elucidated from the ESIMS and NMR spectroscopic data obtained for the active metabolite. The minimum inhibitory concentrations (MICs) of the active compound were determined.
Eight endophytic fungi were isolated from O. dillenii and all except one showed antibacterial activities against at least one of the test bacteria. All extracts were inactive against C. albicans. The most bioactive fungus was identified as Fusarium sp. and the second most active as Aspergillus niger. The structure of the major antibacterial compound of the Fusarium sp. was shown to be the tetramic acid derivative, Equisetin. The MIC's for Equisetin were 8 μg mL(-1) against Bacillus subtilis, 16 μg mL(-1) against Staphylococcus aureus and Methicillin Resistant Staphylococcus aureus (MRSA).
CONCLUSIONS:
O. dillenii, harbors several endophytic fungi capable of producing antimicrobial substances with selective antibacterial properties. By producing biologically active secondary metabolites, such as Equisetin isolated from the endophytic Fusarium sp., the endophytic fungal population may be assisting the host to successfully withstand stressful environmental conditions. Further investigations on the secondary metabolites produced by these endophytes may provide additional drug leads.
J Bioenerg Biomembr. 1993 Oct;25(5):537-45.
Effects of equisetin on rat liver mitochondria: evidence for inhibition of substrate anion carriers of the inner membrane.[Pubmed:
8132493]
The effect of Equisetin, an antibiotic produced by Fusarium equiseti, has been studied on mitochondrial functions (respiration, ATPase, ion transport).
METHODS AND RESULTS:
Equisetin inhibits the DNP-stimulated ATPase activity of rat liver mitochondria and mitoplasts in a concentration-dependent manner; 50% inhibition is caused by about 8 nmol Equisetin/mg protein. The antibiotic is without effect either on the ATPase activity of submitochondrial particles or on the purified F1-ATPase. It inhibits both the ADP- or DNP-activated oxygen uptake by mitochondria in the presence of glutamate+malate or succinate as substrates, but only the ADP-stimulated respiration is inhibited if the electron donors are TMPD+ascorbate. It does not affect the NADH or succinate oxidation of submitochondrial particles. Equisetin inhibits in a concentration-dependent manner the active Ca(2+)-uptake of mitochondria energized both by ATP or succinate without affecting the Ca(2+)-uniporter itself. The antibiotic inhibits the ATP-uptake by mitochondria (50% inhibition at about 8 nmol Equisetin/mg protein) and the Pi and dicarboxylate carrier. It does not lower the membrane potential at least up to 200 nmol/mg protein concentration.
CONCLUSIONS:
The data presented in this paper indicate that Equisetin specifically inhibits the substrate anion carriers of the mitochondrial inner membrane.
Arch Biochem Biophys. 1989 Feb 1;268(2):659-66.
The effect of equisetin on energy-linked reactions in Rhodospirillum rubrum chromatophores.[Pubmed:
2536535]
METHODS AND RESULTS:
Light-induced proton uptake, light-induced carotenoid absorbance shift, photophosphorylation, and hydrolysis of Mg-ATP, Ca-ATP, and PPi in Rhodospirillum rubrum chromatophores are shown to be inhibited by the antibiotic Equisetin.
The Mg- and Ca-ATPase activities of purified F0F1-ATPase are inhibited by Equisetin. In contrast, only the Ca-ATPase activity of purified F1-ATPase is decreased by Equisetin, whereas the Mg-ATPase is stimulated. Both Equisetin and N,N'-dicyclohexylcarbodiimide (DCCD) inhibit the hydrolytic activity of the purified H+-PPase but not the hydrolytic activity of soluble PPase from R. rubrum and yeast. The I50 for the PPi hydrolysis is near 20 microM for both Equisetin and DCCD. The action of Equisetin on membranes is compared to the effect of Triton X-100 and carbonyl cyanide p-trifluoromethoxyhydrazone.
CONCLUSIONS:
On the basis of these new data, Equisetin is proposed to act nonspecifically on membranes and hydrophobic domains of proteins.
Biochem Biophys Res Commun. 2015 May 1;460(2):210-5.
A new enzyme involved in the control of the stereochemistry in the decalin formation during equisetin biosynthesis.[Pubmed:
25770422]
Tetramic acid containing a decalin ring such as Equisetin and phomasetin is one of the characteristic scaffolds found in fungal bioactive secondary metabolites.
METHODS AND RESULTS:
Polyketide (PKS)-nonribosomal peptide synthetase (NRPS) hybrid enzyme is responsible for the synthesis of the polyketide scaffold conjugated with an amino acid. PKS-NRPS hybrid complex programs to create structural diversity in the polyketide backbone have begun to be investigated, yet mechanism of control of the stereochemistry in a decalin formation via a Diels-Alder cycloaddition remains uncertain.
CONCLUSIONS:
Here, we demonstrate that fsa2, which showed no homology to genes encoding proteins of known function, in the fsa cluster responsible for Equisetin and fusarisetin A biosynthesis in Fusarium sp. FN080326, is involved in the control of stereochemistry in decalin formation via a Diels-Alder reaction in the Equisetin biosynthetic pathway.
Chemistry. 2013 Sep 23;19(39):13040-6.
Biomimetic synthesis of equisetin and (+)-fusarisetin A.[Pubmed:
24038394]
(+)-Fusarisetin A belongs to a group of acyl tetramic acid natural products that show potential anticancer activity. Equisetin, a biogenetically related acyl tetramic acid, contains the basic skeleton of (+)-fusarisetin A.
METHODS AND RESULTS:
We proposed that Equisetin and (+)-fusarisetin A share a biosynthetic pathway that starts with naturally occurring (S)-serine and an unsaturated fatty acid. In support of this hypothesis, we have demonstrated that a cyclization sequence involving an intramolecular Diels-Alder reaction followed by a Dieckmann cyclization of polyenoylamino acid yielded Equisetin. The aerobic oxidation of Equisetin, promoted by either Mn(III)/O2 or a reactive oxygen species (ROS) produced by visible-light chemistry, gave peroxyfusarisetin, which could be easily reduced to (+)-fusarisetin A.
CONCLUSIONS:
We report herein detailed information on the biogenetic synthesis of Equisetin and (+)-fusarisetin A.