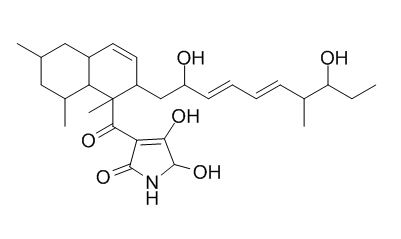
Delaminomycin A
Delaminomycin A is an immunomodulator, it is a cell adhesion inhibitor and extracellular matrix receptor antagonist. Delaminomycin A is active against gram-positive bacteria.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
South African J of Botany2020, 135:50-57
Revista Brasileira de Farmacognosia2024, 34:1156-1165.
Preprints2022, 2022030063.
Biol Pharm Bull.2020, 43(10):1534-1541.
Journal of Functional Foods2022, 96: 105216.
Nat Prod Commun.2017, 12(5):771-778
Journal of Ginseng Research2021, 15 June.
Analytical Letters 2021, 54(4).
J Nat Prod.2019, 82(4):1002-1008
Foods.2021, 10(12):2929.
Related and Featured Products
J Antibiot (Tokyo). 1993 Jul;46(7):1156-62.
Delaminomycins, novel extracellular matrix receptor antagonist. IV. Structure-activity relationships of delaminomycins and derivatives.[Pubmed:
8360111]
Delaminomycin A, Delaminomycin B, Delaminomycin C and their derivatives were prepared and investigated biological activities of them.
METHODS AND RESULTS:
Among these compounds, spiro compounds (A2, B2 and C2) showed stronger inhibitory activity than natural products (A1, B1 and C1) on B16 melanoma cells adhesion assay and Con A-induced proliferation of murine splenic lymphocytes assay. In MLCR and antimicrobial assay, however, A1, B1 and C1 showed more potent inhibitory activity than spiro compounds (A2, B2 and C2). On the other hand, as to C-5' substituents of pyrrolidine ring, the order of inhibitory activity was R = OH > R = OCH3 > R = H on Con A-induced proliferation of murine splenic lymphocytes assay. In MLCR and antimicrobial assay, however, the order of inhibitory activities were R = H > R = OCH3 > R = OH.
CONCLUSIONS:
Inhibitory activities of A4 which was lacked pyrrolidine ring were reduced on B16 melanoma cells adhesion assay and on cytotoxicity against tumor cells in vitro in comparison with those of A1.
J Antibiot (Tokyo). 1993 Jun;46(6):979-84.
Delaminomycins, novel nonpeptide extracellular matrix receptor antagonist and a new class of potent immunomodulator. II. Physico-chemical properties and structure elucidation of delaminomycin A.[Pubmed:
8393852]
METHODS AND RESULTS:
Delaminomycins, novel extracellular matrix receptor antagonists, have been isolated from a culture broth of Streptomyces albulus MJ202-72F3. The structure of Delaminomycin A was determined to be 3-[[2-[(3E,5E)-2,8-dihydroxy-7-methyl-3,5-decadienyl]- 1,6,8- trimethyl-1,2,4a,5,6,7,8,8a-octahydro-1-naphthyl]carbonyl]-5- hydroxypyrrolidine-2,4-dione by analyses of spectral properties and chemical studies.
US 5527820 A[P]. 1996.
Antibiotics having immunosuppressive activity, delaminomycins and processes for the production of the same[Reference:
WebLink]
As new antibiotics are obtained delaminomycins A, B and C having a formula (I) or (I') shown below, or salts thereof.
METHODS AND RESULTS:
Delaminomycins preferentially inhibit T cells and are useful as immunosuppressants, antineoplastic agents and antibacterial agents. ##STR1## wherein X is a hydroxyl group for Delaminomycin A, a methoxy group for delaminomycin B and a hydrogen atom for delaminomycin C.