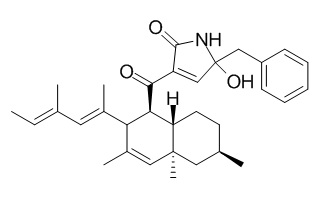
Oteromycin
Oteromycin is a inhibitor of HIV-1 integrase.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Journal of Molecular Liquids2022, 364:120062.
PLoS One.2015, 10(5):e0127060
Korean J. Food Preserv. 2021, 28(6):846-856.
Oxid Med Cell Longev.2022, 2022:5888636.
Int J Cosmet Sci.2023, 45(2):155-165.
Arch Biochem Biophys.2024, 759:110111.
J Anal Methods Chem.2024, 2024:7703951.
Mal J Med Health Sci.2024, 20(SUPP5):151-156.
Braz J Med Biol Res.2021, 54(12):e11183.
J.Food Processing & Preservation2022, jfpp.16666
Related and Featured Products
Antivir Chem Chemother. 1999 Mar;10(2):63-70.
Isolation and characterization of novel human immunodeficiency virus integrase inhibitors from fungal metabolites.[Pubmed:
10335400]
We have identified a series of novel inhibitors of human immunodeficiency virus type 1 (HIV-1) integrase by randomly screening natural product extracts using an in vitro biochemical assay designed to identify inhibitors of integrase-catalysed strand transfer.
METHODS AND RESULTS:
Equisetin recovered from the fungus Fusarium heterosporum and a novel enantiomeric homologue of equisetin from Phoma sp. were isolated as inhibitors of HIV-1 integrase in vitro. Two additional analogues, a novel decalin derivative, integric acid, and Oteromycin were also discovered to be inhibitors of integrase. Equisetin and related compounds inhibit 3' end-processing and strand transfer as well as disintegration catalysed by either the full-length enzyme or the truncated integrase core domain (amino acids 50-212). These compounds also inhibit strand transfer reactions catalysed by stable complexes assembled in vitro and integration reactions catalysed by pre-integration complexes isolated from HIV-1-infected cells. The compounds described in this report are structurally novel and mechanistically distinct from many previously described inhibitors of HIV-1 integrase.
CONCLUSIONS:
These results demonstrate the utility of using an appropriately configured assay to identify compounds that are effective post-assembly and the potential of isolating novel integrase inhibitors from complex natural product extracts.
Tetrahedron Letters,2013,54(6):506–511.
First total synthesis of oteromycin utilizing one-pot four-step cascade reaction strategy[Reference:
WebLink]
The first total synthesis of Oteromycin was investigated.
METHODS AND RESULTS:
Our previously reported convergent strategy for the synthesis of α-acyl-γ-hydroxy-γ-lactams was first applied for the total synthesis, however, the final deprotection of the methoxyaminal moiety could not be achieved since an unexpected intramolecular Diels–Alder (IMDA) reaction occurred. Therefore, a novel one-pot four-step cascade reaction starting from α-selenolactam was investigated.
CONCLUSIONS:
The efficient synthetic strategy was successfully developed to afford the desired Oteromycin, and its complete structure elucidation including the stereochemistry at C24 position was also accomplished.
Proceedings of the Symposium on Progress in Organic Reactions and Syntheses. 2009.
Synthetic Study of HIV Integrase Inhibitor Oteromycin[Reference:
WebLink]
Oteromycin is a HIV integrase inhibitor isolated from fungi MF5810 and MF5811, and has attracted a lot of attention for it's constructive features, decalin skeleton and alpha,beta-unsaturated-alpha-acyl-gamma-hydroxylactam moiety.
METHODS AND RESULTS:
In the initial stage of the total synthesis, the novel synthetic method of alpha,beta-unsaturated-alpha-acyl-gamma-hydroxylactam moiety was developed utilizing the catalytic acid-mediated dehydrogenation of alpha-acyl-gamma-hydroxylactam by DDQ. We succeeded in establishing the novel synthetic method, lanched synthesis of the decalin skeleton of Oteromycin. As a key step for the construction of the decalin skeleton, an intramolecular Diels-Alder (IMDA) reaction was adopted.
CONCLUSIONS:
The synthesis of the decalin equipped with all stereogenic centers has been achieved, starting from commercially available (+)-citronellal.