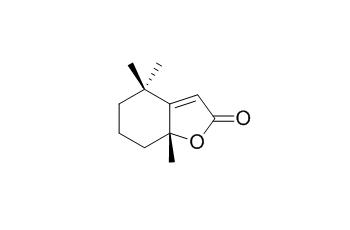
Dihydroactinidiolide
Dihydroactinidiolide, a high light-induced β-carotene derivative that can regulate gene expression and photoacclimation in Arabidopsis. Dihydroactinidiolide has shown to be a potent phytotoxin at low concentration, it may have interesting potential anticancer activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int Immunopharmacol.2023, 123:110572.
Korean j.of Pharm.2017, 70-76
Environ Toxicol.2019, 34(4):513-520.
Pharmacognosy Magazine2018, 14(56):418-424
J.Korean Society of Grassland&Forage Science2023, 43(3):138-147.
ACS Food Sci. Technol.2023, 3(2):273-282.
Appl. Sci. 2021, 11(17),7829
Int J Mol Med.2015, 35(5):1237-45
Journal of Herbal Medicine2024, 48:100950
University of Burgos2024, ssrn.4795441.
Related and Featured Products
Nat Prod Commun. 2010 Jul;5(7):1127-32.
Antiproliferative and cytotoxic effects on malignant melanoma cells of essential oils from the aerial parts of Genista sessilifolia and G. tinctoria.[Pubmed:
20734956]
Genista species (family Leguminosae) show interesting biological properties.
METHODS AND RESULTS:
In this paper we describe the biological activity of the essential oils extracted from the aerial parts of G. sessilifolia DC. and G. tinctoria L. against M14 human melanoma cells, testing several biochemical parameters, such as cell vitality, cell membrane integrity and genomic DNA fragmentation. In addition, we report for the first time the study of the composition of the essential oil obtained from G. tinctoria. The most abundant components of the oil were carbonylic compounds such as (E)-beta-ionone (9.1%), Dihydroactinidiolide (7.3%), nonanal (5.1%) and hexahydrofarnesylacetone (4.3%).
CONCLUSIONS:
The essential oils from aerial parts of both G. sessilifolia and G. tinctoria showed interesting potential anticancer activity, suggesting the presence of active compounds.
Experientia, 1981, 37(11):1133-1133.
Dihydroactinidiolide—a potent growth inhibitor from Eleocharis coloradoensis (spikerush)[Reference:
WebLink]
Dihydroactinidiolide has been isolated fromEleocharis coloradoensis, along with p-coumaric acid, ferulic acid and luteolin, and shown to be a potent phytotoxin at low concentration.
Mol Plant. 2014 Jul;7(7):1248-51.
Dihydroactinidiolide, a high light-induced β-carotene derivative that can regulate gene expression and photoacclimation in Arabidopsis.[Pubmed:
24646629 ]
Dihydroactinidiolide, a high light-induced β-carotene derivative that can regulate gene expression and photoacclimation in Arabidopsis.
Yakugaku Zasshi. 1969 Dec;89(12):1729-31.
Studies on the essential oil of green tea. I. Isolation of dihydroactinidiolide and p-vinylphenol.[Reference:
WebLink]
METHODS AND RESULTS:
Numerous compounds have been identified in the essential oil of green tea but in the present series of experiments, several unknown components were isolated from the high-boiling fraction of the essential oil of green tea by the use of gas, column, and thin-layer chromatography.
CONCLUSIONS:
Two of these components were identified as Dihydroactinidiolide and p-vinylphenol, obtained earlier from the Actinidia polygama and black tea. The identification was based on infrared, ultraviolet, mass, and nuclear magnetic resonance spectral data.