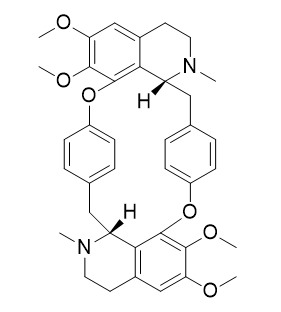
Cycleanine
The biological screening of cycleanine and the root bark alkaloidal extract revealed potent antibacterial, antifungal, antiplasmodial, and cytotoxic activities. Cycleanine, like its isomer – tetrandrine isolated from T. subcordata, could be a potential new anti-ovarian cancer agent acting through the apoptosis pathway. It also shows antiplasmodial activities against Plasmodium falciparum 3D7 with IC50 values of 0.08 µM.Cycleanine may have anti-inflammatory activity. Cycleanine markedly inhibited Na(+),K(+)-ATPase activity with an IC(50) value of 6.2 x 10(-4)M. It slightly inhibited Mg(2+)-ATPase, H(+)-ATPase, and Ca(2+)-ATPase, it might interact with the enzyme in Na.E(1)-P form and prevents the reaction step from Na.E(1)-P to E(2)-P.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Sci Rep. 2018, 1-9
J. Mater. Life Sci.2024, 3:2:78-87
Toxins (Basel).2023, 15(3):231.
QASCF2022, 14(4).
Phytomedicine.2023, 114:154813.
Hortic Res.2023, 10(9):uhad154.
Applied Biological Chemistry2023, 66:8
Oncotarget.2017, 8(53):90925-90947
Comput Biol Chem.2019, 83:107096
Int J Med Sci.2024, 21(15):2883-2896
Related and Featured Products
Planta Medica, 2016, 81(S 01):S1-S381.
Cytotoxicity effects and apoptosis induction by cycleanine and tetrandrine.[Reference:
WebLink]
Ovarian cancer remains one of the main causes of death in all gynecologic malignancies [1]. Natural products continue to be important sources of clinically approved anti-cancer drugs [2, 3]. Triclisia subcordata Oliv (Menispermeaceae) is a medicinal plant traditionally used for the treatment of various diseases [4], including cancer, in West Africa. This study aims to evaluate the in vitro anti-ovarian cancer activities of the crude extracts and the isolated components in T. subcordata.
METHODS AND RESULTS:
The ethanol extract of T. subcordata and its fractions (crude alkaloids) were screened for in vitro anti-ovarian cancer activities on Ovcar-8, Ovcar-4, A2780, and Igrov-1 ovarian cancer cell lines using the Sulforhodamine B assay method to measure cell growth. Bioassay-guided fractionation using silica gel column chromatography and HPLC were used to isolate the bioactive compound, whose identity and structure was identified by NMR and LC-MS techniques. Caspase and PARP cleavage assays were used to detect apoptotic activities. The effect of isolated pure compounds on cell cycle and apoptosis was analyzed by flow cytometry. The crude alkaloids showed the strongest activity in cell growth assays on A2780 and Ovcar-8 cell lines (IC50 < 2.4 µg/mL). A bisbenzylisoquinoline alkaloid-Cycleanine was isolated using HPLC and identified by MS and NMR analyses. The IC50values of Cycleanine and tetrandrine ranged from 7 to 14µM on A2780, Ovcar-8, Ovcar-4 and Igrov-1 ovarian cancer cell lines. The IC50 of Cycleanine on human normal ovarian surface epithelial cells was 35 ± 1µM hinting at modest selectivity towards cancer cells. Both Cycleanine and tetrandrine caused apoptosis as shown by activation of caspases 3/7 and cleavage of poly (ADP) ribose polymerase (PARP) to form PARP-I. The percentage of Ovcar-8 cells in subG1 phase increased after exposure of Cycleanine and tetrandrine to cells for 48h compared to control.
CONCLUSIONS:
In conclusion, Cycleanine, like its isomer – tetrandrine isolated from T. subcordata, could be a potential new anti-ovarian cancer agent acting through the apoptosis pathway.
Nat Prod Commun. 2015 Sep;10(9):1541-2.
Anti-malarial Activity of Isoquinoline Alkaloids from the Stem Bark of Actinodaphne macrophylla.[Pubmed:
26594753]
METHODS AND RESULTS:
Seven isoquinoline alkaloids isolated from the bark of Actinodaphne macrophylla in this study demonstrated in vitro antiplasmodial activities against Plasmodium falciparum 3D7 with IC50 values of 0.08 µM, 0.05 µM, 1.18 µM, 3.11 µM, 0.65 µM, 0.26 µM, and 1.38 µM for Cycleanine, 10-demethylxylopinine, reticuline, laurotetanine, bicuculine, α-hydrastine and anolobine, respectively, which are comparable with the reference standard, chloroquine.
CONCLUSIONS:
10-Demethylxylopinine was found to be the most active of these compounds.
J Ethnopharmacol. 2004 Aug;93(2-3):331-5.
Antibacterial, antifungal, antiplasmodial, and cytotoxic activities of Albertisia villos[Pubmed:
15234773 ]
Albertisia villosa (Menispermaceae) is a subtropical medicinal plant that is widely used in traditional African medicines against various diseases.
METHODS AND RESULTS:
Three known bisbenzylisoquinoline alkaloids; Cycleanine, cocsoline, and N-desmethylCycleanine have been identified. Cycleanine, the most abundant (85%) of all identified bisbenzylisoquinoline alkaloids, accounts for all of the activity of the crude drug. The biological screening of Cycleanine and the root bark alkaloidal extract revealed potent antibacterial, antifungal, antiplasmodial, and cytotoxic activities.
CONCLUSIONS:
These results may partly explain and support the use of Albertisia villosa root barks for the treatment of malaria and other infectious diseases in traditional Congolese medicine.
Biochemical Pharmacology, 2003, 66(3):379-385.
Inhibition of Na+,K+-ATPase by the extract of Stephania cephararantha Hayata and bisbenzylisoquinoline alkaloid cycleanine, a major constituent.[Pubmed:
12907236 ]
The Stephania cephararantha HAYATA extract, and its constituent bisbenzylisoquinoline alkaloids, such as Cycleanine, cepharanthine, isotetrandrine, berbamine, homoaromoline, and cepharanoline were studied for effects on Na(+),K(+)-ATPase activity.
METHODS AND RESULTS:
The S. cephararantha HAYATA extract inhibited Na(+),K(+)-ATPase activity with an apparent IC(50) value of 540 microg/mL. Cycleanine markedly inhibited Na(+),K(+)-ATPase activity with an IC(50) value of 6.2 x 10(-4)M. It slightly inhibited Mg(2+)-ATPase, H(+)-ATPase, and Ca(2+)-ATPase. No effects on alkaline and acid phosphatase activities were observed. The inhibition by isotetrandrine, homoaromoline, cepharanthine, and berbamine was less marked, and cepharanoline showed no effect. Five synthetic analogues of cepharanthine slightly inhibited the activity. The mechanism of inhibition by Cycleanine on Na(+),K(+)-ATPase activity was examined in detail, and the following results were obtained in the overall reaction: (1) the mode of inhibition was noncompetitive with respect to ATP; (2) the degree of inhibition was decreased with a decrease of K(+) concentration; (3) it was not affected by Na(+) concentration; (4) the inhibition mechanism was different from that of ouabain. The activity of K(+)-dependent p-nitrophenyl phosphatase, a partial reaction of Na(+),K(+)-ATPase, did not appear to have been inhibited by Cycleanine in the reaction mixture containing 15 mM K(+) (optimum condition). However, Cycleanine increased the K(0.5) value for K(+) and reduced the K(i) values for Na(+) and ATP, in K(+)-dependent p-nitrophenyl phosphatase.
CONCLUSIONS:
Cycleanine might interact with the enzyme in Na.E(1)-P form and prevents the reaction step from Na.E(1)-P to E(2)-P.
Biochem Pharmacol. 1993 Dec 3;46(11):1887-92.
Inhibitory effect of bisbenzylisoquinoline alkaloids on nitric oxide production in activated macrophages.[Pubmed:
7505581]
Bisbenzylisoquinoline (BBI) alkaloids are anti-inflammatory constituents of plants of the families Menispermaceae and Ranunculaceae, which have been used as folk remedies in Japan and China.
METHODS AND RESULTS:
Five BBI alkaloids (cepharanthine, chondocurine, Cycleanine, isotetrandrine and tetrandrine) were tested for suppressive effect on in vitro nitric oxide (NO) production by lipopolysaccharide-stimulated peritoneal macrophages, which were induced with thioglycollate or bacillus Calmette-Guerin in mice.
CONCLUSIONS:
All these BBI alkaloids significantly suppressed NO production at 5 micrograms/mL. Cepharanthine, isotetrandrine and Cycleanine were slightly more inhibitory than tetrandrine and chondocurine. The suppression persisted for at least 48 hr. As NO is one of the critical mediators in inflammation, these results may explain some aspects of the anti-inflammatory mechanisms of BBI compounds.
Bioorg Med Chem Lett. 2018 May 15;28(9):1652-1656.
Synthesis of (aminoalkyl)cycleanine analogues: cytotoxicity, cellular uptake, and apoptosis induction in ovarian cancer cells.[Pubmed:
29588214]
Our previous studies demonstrated that Cycleanine, a macrocyclic bisbenzylisoquinoline (BBIQ) alkaloid, showed potent anti-ovarian cancer activity via apoptosis induction.
METHODS AND RESULTS:
Here, we synthesized two novel (aminoalkyl)Cycleanine analogues (2 and 3) through a simple and efficient two-step reaction starting from Cycleanine isolated from Triclisia subcordata Oliv. These analogues showed greater potency than the unmodified Cycleanine in three human ovarian cancer cell lines. Both 2 and 3 induced apoptosis in ovarian cancer cells by activations of caspases 3/7, cleavage of PARP, increase in subG1 cell cycle phase and in the percentage of apoptotic cells. Further confocal fluorescence microscopy analysis confirmed the cellular uptake of alkaloids in ovarian cancer cells by using the unique (alkynyl)Cycleanine (3) via click chemistry reaction.
CONCLUSIONS:
Our results suggest that Cycleanine could be a hit compound for the future development in attacking ovarian cancer.
Phytochemistry, 1980, 19(8):1837-1840.
Cycleanine from Synclisia scabrida: conformational information from the 1H NMR spectrum at 300 MHz.[Reference:
WebLink]
METHODS AND RESULTS:
Cycleanine, a bisbenzylisoquinoline alkaloid, has been isolated from the roots of Synclisia scabrida. The 1H NMR spectrum at 300 MHz reveals that, in chloroform solution, Cycleanine has a conformation whereby ring B partly shields ring C′ and ring C is similarly influenced by ring B′.