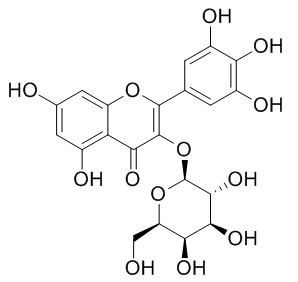
Myricetin 3-O-galactoside
Myricetin 3-O-galactoside has cytotoxicity, antioxidant, anti-genotoxic, antinociceptive and anti-inflammatory effects, the effects are related to peripheral inhibition of nitric oxide synthesis, mainly inducible nitric oxide synthase (iNOS).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Pharmaceutics.2021, 13(7):1028.
Srinagarind Medical Journal2019, 34(1)
J Korean Med Ophthalmol Otolaryngol Dermatol2023, 36(1):21-39.
Cell Death Differ.2021, 1-8.
Nutr Res Pract.2020, 14(3):203-217.
Appl. Sci.2020, 10(23), 8729
Natural Product Communications2021, 16(9):1-10.
Molecules.2021, 26(19):6032.
J of Engineering Science&Technology2018, 13(9):2820-2828
J Ethnopharmacol.2022, 291:115159.
Related and Featured Products
Toxicol In Vitro. 2008 Apr;22(3):567-81.
In vitro antioxidant and antigenotoxic potentials of myricetin-3-o-galactoside and myricetin-3-o-rhamnoside from Myrtus communis: modulation of expression of genes involved in cell defence system using cDNA microarray.[Pubmed:
18222061]
Antioxidant activity of Myricetin 3-O-galactoside and myricetin-3-o-rhamnoside, isolated from the leaves of Myrtus communis, was determined by the ability of each compound to inhibit xanthine oxidase activity, lipid peroxidation and to scavenge the free radical 1,1-diphenyl-2-picrylhydrazyl.
METHODS AND RESULTS:
Antimutagenic activity was assessed using the SOS chromotest and the Comet assay. The IC50 values of lipid peroxidation by Myricetin 3-O-galactoside and myricetin 3-o-rhamnoside are respectively 160 microg/ml and 220 microg/ml. At a concentration of 100 microg/ml, the two compounds showed the most potent inhibitory effect of xanthine oxidase activity by respectively, 57% and 59%. Myricetin 3-o-rhamnoside was a very potent radical scavenger with an IC50 value of 1.4 microg/ml. Moreover, these two compounds induced an inhibitory activity against nifuroxazide, aflatoxine B1 and H2O2 induced mutagenicity.
CONCLUSIONS:
The protective effect exhibited by these molecules was also determined by analysis of gene expression as response to an oxidative stress using a cDNA micro-array. Myricetin 3-O-galactoside and myricetin 3-o-rhamnoside modulated the expression patterns of cellular genes involved in oxidative stress, respectively (GPX1, TXN, AOE372, SEPW1, SHC1) and (TXNRD1, TXN, SOD1 AOE372, SEPW1), in DNA damaging repair, respectively (XPC, LIG4, RPA3, PCNA, DDIT3, POLD1, XRCC5, MPG) and (TDG, PCNA, LIG4, XRCC5, DDIT3, MSH2, ERCC5, RPA3, POLD1), and in apoptosis (PARP).
Nat Prod Res. 2013;27(17):1569-75.
Betula pendula Roth leaves: gastroprotective effects of an HPLC-fingerprinted methanolic extract.[Pubmed:
23163340]
In this study, a methanolic extract of Betula pendula leaves (BLE) was investigated for its gastroprotective effects against 90% ethanol-induced ulcer in rats.
METHODS AND RESULTS:
Oral pretreatment of rats with BLE (100, 200 and 400 mg kg(- 1)) significantly reduced the incidence of gastric lesions induced by ethanol administration as compared with misoprostol (0.50 mg kg(- 1)). Furthermore, BLE inhibited the increase in malondialdehyde (MDA) and prevented depletion of total sulhydryl and non-protein sulhydryl groups in rat stomach homogenate when compared with ethanol group. With regard to the effect of lipid peroxidation in vitro, BLE showed the ability to reduce methyl linoleate autoxidation.
CONCLUSIONS:
Chemical characterisation of the main biologically active constituents of BLE was also achieved by means of high-performance liquid chromatography with photodiode array and mass spectrometry detection, showing the presence of Myricetin 3-O-galactoside, quercetin glycosides, kaempferol glycosides.
Asian Journal of Plant Sciences, 2012, 11(3): 124-30.
Cytotoxicity and Suppressive Effect of Leaves of Mimusops laurifolia on Carbon Tetrachloride-induced Liver Injury in Rats and its Bioactive Constituents[Reference:
WebLink]
Since the genus Mimusops is one of the important genera in the Indian traditional medicine, and is represented in Egypt with species; Mimusops laurifolia (Forssk.) Friis., thus the plant is selected for our investigation to reveal its biological activities and phytochemically analyze its bioactive fractions.
METHODS AND RESULTS:
The ethanolic extract of its leaves (LEE) and its different fractions: n-hexane (HF), chloroform (CF), ethyl acetate (EAF) and n-butanol (BF) were evaluated for in vivo hepatoprotective activity against CC14 induced hepatic cell damage in rats and for in vitro cytotoxicity against human liver cancer cell line (HEPG2); consequently the bioactive constituents were defined whereby EAF evidenced statistically significant hepatoprotection. Moreover, HF and α-amyrin (major compound isolated from HF) showed promising cytotoxicity against HEPG2. Structures of isolated compounds were established on the basis of physicochemical properties and spectral analysis. The bioactive fractions were examined for the isolation of 14 compounds for the first time from Mimusops laurifolia (Forssk.) Friis. From the lipophilic fractions: Lupeol acetate, α-amyrin, chondrillasterol, oleanolic acid, chondr111 asterol-3-O-β-D-galactoside, meamsitrin, myricetin and quercetin were isolated. While, from EAF: mearnsitrin, myricitrin, myricetin- 3 - O -β-D -g alactoside(Myricetin 3-O-galactoside), quercetin- 3 - O - β-D-g lucoside, rutin and myncetin-3-0-β- D-glucuronide were isolated.
CONCLUSIONS:
Leaves of Mimusops laurifolia (Forssk.) Friis can be considered as a natural medicinal plant with a potential anticancer and hepatoprotection due to its bioactive ingredients in both HF and EAF, respectively.
J Nat Med. 2015 Jul;69(3):303-12.
Antinociceptive and anti-inflammatory effects of myricetin 3-O-β-galactoside isolated from Davilla elliptica: involvement of the nitrergic system.[Pubmed:
25894075 ]
We aimed to study the antinociceptive effects of myricetin 3-O-β-galactoside (Myricetin 3-O-galactoside,Mi), a substance isolated from the hydroalcoholic extract of Davilla elliptica.
METHODS AND RESULTS:
This study examined male Swiss mice, inducible nitric oxide synthase C57B16/J knockout mice (iNOS(-/-)), and their corresponding wild type (WT). Formalin and tail-flick tests were used to evaluate the nociceptive threshold, and the carrageenan-induced paw edema test was used as a model for inflammation. The following drugs were administered to investigate the involvement of the nitrergic and opioidergic systems: L-NAME, a nonspecific nitric oxide synthase (NOS) inhibitor; L-arginine (L-Arg), a precursor for the synthesis of nitric oxide (NO); D-arginine (D-Arg), an inactive isomer for the synthesis of NO; aminoguanidine (Am), an inducible nitric oxide synthase (iNOS) inhibitor; and naloxone, a nonselective antagonist of opioid receptors. The results showed that oral pretreatment with Mi caused a dose-dependent inhibition of the inflammatory phase of the formalin test and did not alter motor performance. Intraperitoneal injection of L-NAME caused a reduction in the licking time during the second phase of the formalin test. The administration of L-Arg (but not D-Arg) reversed the antinociceptive effect of L-NAME. Furthermore, pre-administration of aminoguanidine potentiated the antinociceptive effect. Mi did not cause an antinociceptive effect in iNOS knockouts and led to a reduction in the nitrite concentration in the paws of mice. Carrageenan-induced paw edema was reduced in Swiss mice and WT mice when compared to iNOS(-/-) mice. Pre-administration of naloxone (NLX) did not reverse the antinociceptive effect of Mi, excluding the opioidergic system as a mediator of the antinociceptive effect.
CONCLUSIONS:
Thus, the results suggest that the antinociceptive and anti-inflammatory effects of myricetin 3-O-β-galactoside(Myricetin 3-O-galactoside) are related to peripheral inhibition of nitric oxide synthesis, mainly iNOS.