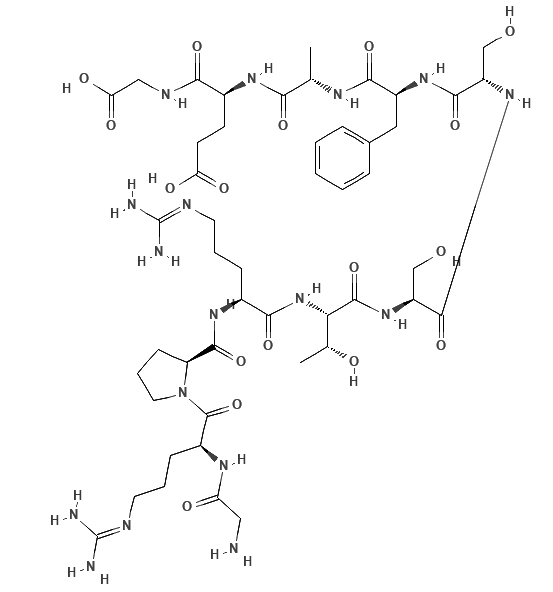
Crosstide
Crosstide is a peptide analog of glycogen synthase kinase α/β fusion protein sequence which is a substrate for Akt.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Chem.2024, 458:140201.
Pharmaceuticals (Basel).2024, 17(4):462.
Naunyn Schmiedebergs Arch Pharmacol.2017, 390(10):1073-1083
Pharmaceuticals (Basel).2024, 17(4):442.
Drug Chem Toxicol.2020, 1-12.
Research Square2021, March 3rd.
J Biochem Mol Toxicol.2021, 35(5):e22731.
Int J Mol Sci.2020, 21(9):3392.
Food Chem.2017, 228:301-314
Drug Dev Res.2020, doi: 10.1002
Related and Featured Products
J Virol. 2007 Feb;81(3):1186-1194.
Rhinovirus activates interleukin-8 expression via a Src/p110beta phosphatidylinositol 3-kinase/Akt pathway in human airway epithelial cells[Pubmed:
17121804]
Rhinovirus (RV) is responsible for the majority of common colds and triggers exacerbations of asthma and chronic obstructive lung disease. We have shown that RV serotype 39 (RV39) infection activates phosphatidylinositol 3 (PI 3)-kinase and the serine threonine kinase Akt minutes after infection and that the activation of PI 3-kinase and Akt is required for maximal interleukin-8 (IL-8) expression. Here, we further examine the contributions of Src and PI 3-kinase activation to RV-induced Akt activation and IL-8 expression. Confocal fluorescent microscopy of 16HBE14o- human bronchial epithelial cells showed rapid (10-min) colocalization of RV39 with Src, p85alpha PI 3-kinase, p110beta PI 3-kinase, Akt and Cit-Akt-PH, a fluorescent Akt pleckstrin homology domain which binds PI(3,4,5)P(3). The chemical Src inhibitor PP2 {4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo [3,4-d]pyrimidine} and the PI 3-kinase inhibitor LY294002 each inhibited Akt phosphorylation and the colocalization of RV39 with Akt. Digoxigenin-tagged RV coprecipitated with a Crosstide kinase likely to be Akt, and inhibition of Src blocked kinase activity. Digoxigenin-tagged RV39 colocalized with the lipid raft marker ceramide. In 16HBE14o- and primary mucociliary differentiated human bronchial epithelial cells, inhibition of Src kinase activity with the Src family chemical inhibitor PP2, dominant-negative Src (K297R), and Src small interfering RNA (siRNA) each inhibited RV39-induced IL-8 expression. siRNA against p110beta PI 3-kinase also inhibited IL-8 expression. These data demonstrate that, in the context of RV infection, Src and p110beta PI 3-kinase are upstream activators of Akt and the IL-8 promoter and that RV colocalizes with Src, PI 3-kinase, and Akt in lipid rafts.
Biochim Biophys Acta. 2005 Oct 10;1725(3):340-347.
Activation of a GST-tagged AKT2/PKBbeta[Pubmed:
15890450]
The protein kinase AKT is a key regulator for cell growth, cell survival and metabolic insulin action. However, the mechanism of activation of AKT in vivo, which presumably involves membrane recruitment of the kinase, oligomerization, and multiple phosphorylation events, is not fully understood. In the present study, we have expressed and purified dimeric GST-fusion proteins of human protein kinase AKT2 (DeltaPH-AKT2) in milligram quantities via the baculovirus expression system. Treatment of virus-infected insect cells with the phosphatase inhibitor okadaic acid (OA) led to phosphorylation of the two regulatory phosphorylation sites, Thr309 and Ser474, and to activation of the kinase. Likewise, phosphorylation of Thr309 in vitro by recombinant PDK1 or mutation of Thr309 and Ser474 to acidic residues rendered the kinase constitutively active. However, even though the specific activity of our AKT2 was increased 15-fold compared to previous reports, GST-mediated dimerization alone did not lead to an activation of the kinase. Whereas both mutagenesis and phosphorylation led to an increase in the turnover number of the enzyme, only the latter resulted in a marked reduction (20-fold) of the apparent Km value for the exogenous substrate Crosstide, indicating that this widely used mutagenesis only partially mimics phosphorylation. Kinetic analysis of GST-AKT2 demonstrates that phosphorylation of Thr309 in the activation loop of the kinase is largely responsible for the observed reduction in Km and for a subsequent 150-fold increase in the catalytic efficiency (k(cat)/Km) of the enzyme. Highly active AKT2 constructs were used in autophosphorylation reactions in vitro, where inactive AKT2 kinases served as substrates. As a matter of fact, we found evidence for a minor autophosphorylation activity of AKT2 but no significant autophosphorylation of any of the two regulatory sites, Thr309 or Ser474.
Protein Expr Purif . 2005 Sep;43(1):44-56.
Improved yields for baculovirus-mediated expression of human His(6)-PDK1 and His(6)-PKBbeta/Akt2 and characterization of phospho-specific isoforms for design of inhibitors that stabilize inactive conformations[Pubmed:
16084396]
PDK1 and PKB/Akt have a pleckstrin homology (PH) domain at the C-terminus and N-terminus, respectively, which stabilizes an unphosphorylated, autoinhibited conformation. Binding of the PH domain to a phospholipid second messenger causes relief of autoinhibition, which results in kinase phosphorylation and activation. Baculovirus-mediated expression in Sf9 insect cells of both His(6)-PDK1 and His(6)-PKBbeta/Akt2 were optimized, which significantly improved the yields (5-fold) of the affinity purified enzymes over previously reported values. Isoelectric focusing (IEF) and Western analyses indicated that the apparent V(max)=192+/-13 U/mg and K(m) (PDK-Tide)=55+/-10 microM of purified His(6)-PDK1 results from a mixture of at least three different phospho-specific isoforms (pI values of 6.8, 6.5, and 6.4). A purely unphosphorylated isoform of His(6)-PDK1 (pI=6.8) was generated by treatment with lambda protein phosphatase (lambdaPP), which decreased V(max) to 2.4+/-0.4 U/mg and increased K(m) (PDK-Tide) to 217+/-61 microM. Isoelectric focusing and Western analyses indicated that the apparent V(max)=0.21+/-0.03 U/mg and K(m) (Crosstide)=87+/-30 microM of purified His(6)-PKBbeta/Akt2 results from a mixture of the enzyme monophosphorylated either at Ser-474 ( approximately 90%) or at Thr-309 ( approximately 10%). A purely unphosphorylated isoform of His(6)-PKBbeta/Akt2 (pI=6.4) was generated by treatment with lambdaPP, which decreased V(max) approximately 2-fold. The optimization of high-level production and detailed characterization of purified and lambdaPP-treated His(6)-PDK1 and His(6)-PKBbeta/Akt2 will facilitate detailed structural and kinetic studies aimed at understanding the mechanism of second messenger-induced activation.
Carasinol D
Catalog No: CFN95044
CAS No: 1072797-66-0
Price: $333/5mg
2''-O-acetylsaikosaponin A
Catalog No: CFN95085
CAS No: 102934-42-9
Price: $318/5mg
(1E)-3-methoxy-8,12-epoxygermacra-1,7,10,11-tetraen-6-one
Catalog No: CFN95219
CAS No: 1393342-06-7
Price: $413/5mg
Isoedultin
Catalog No: CFN95273
CAS No: 43043-08-9
Price: $318/5mg
Apigenin 4'-O-(2'',6''-di-O-E-p-coumaroyl)glucoside
Catalog No: CFN95276
CAS No: 71781-79-8
Price: $318/10mg
Eriodictyol 7-O-glucuronide
Catalog No: CFN95290
CAS No: 125535-06-0
Price: $318/10mg
Isosaponarin 2''-O-glucoside (Isovitexin-2''-4'-di-O-beta-D-glucoside)
Catalog No: CFN95296
CAS No: 63316-27-8
Price: $318/10mg
4'-Hydroxy-5,7,3'-trimethoxyflavone
Catalog No: CFN95398
CAS No: 1239-68-5
Price: $318/5mg
3',4',5',5,7-Pentamethoxyflavanone
Catalog No: CFN95414
CAS No: 479672-30-5
Price: $218/20mg
12beta-Acetoxy-3,7,11,15,23-pentaoxo-lanost-8,20-dien-26-oic acid
Catalog No: CFN95505
CAS No: 1309931-91-6
Price: $318/5mg