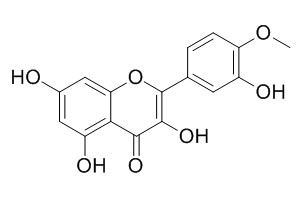
Tamarixetin
Tamarixetin has vasodilator effects in rat isolated vessels. Tamarixetin has cytotoxic against leukemia cells and in particular P-glycoprotein- overexpressing K562/ADR cells, it inhibits proliferation in a concentration- and time-dependent manner, induces apoptosis and blocked cell cycle progression at G2 -M phase.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Indian J Pharm Sci.2024, 86(2):736-741.
J of the Korean Society of Cosmetics and Cosmetology2018, 399-406
Microchemical Journal2018, 137:168-173
Pharmacognosy Magazine2018, 14(56):418-424
Front Microbiol.2021, 12:736780.
Cell Signal.2024, 124:111467.
Molecules.2020, 25(11):2599.
Plos One.2019, 15(2):e0220084
J Agric Food Chem.2024, 72(15):8784-8797.
Chemistry of Natural Compounds2018, 54(3):572-576
Related and Featured Products
J Agric Food Chem. 2012 Sep 12;60(36):9292-7.
Competition between ascorbate and glutathione for the oxidized form of methylated quercetin metabolites and analogues: tamarixetin, 4'O-methylquercetin, has the lowest thiol reactivity.[Pubmed:
22860763]
Quercetin (Q) is a bioactive compound with excellent antioxidant activity. However, the thiol reactivity of its oxidation product (oxQ) forms a disadvantage. The aim of the present study was to decrease this thiol toxicity.
METHODS AND RESULTS:
We found that methylated Q metabolites displayed lower thiol reactivity than Q. The most effective was Tamarixetin, 4'O-methylquercetin (4'MQ), that has a corresponding oxidation product (ox4'MQ) with thiol reactivity 350 times lower than oxQ. The endogenous metabolism of Q to 4'MQ might be a physiological way to safely benefit from the antioxidant potential of Q in vivo.
CONCLUSIONS:
Our results were explained with Pearson's HSAB concept and corroborated by quantum molecular calculations that revealed a strong correlation between the relative thiol reactivity and the lowest unoccupied molecular orbital (LUMO). The polarity of the molecule and the π-π interaction between the AC- and the B-ring appeared to determine the LUMO and the thiol reactivity of the oxidation product.
Brit. J. Pharmacol., 2000, 131: U11-U11.
Vasodilator effects of quercetin and its metabolites, isorhamnetin and tamarixetin, in rat isolated vessels.[Reference:
WebLink]
METHODS AND RESULTS:
Vasodilator effects of quercetin and its metabolites, isorhamnetin and Tamarixetin, in rat isolated vessels.
J Mol Histol . 2019 Aug;50(4):343-354.
Tamarixetin protects against cardiac hypertrophy via inhibiting NFAT and AKT pathway[Pubmed:
31111288]
Abstract
Cardiac hypertrophy is a compensatory response in reaction to mechanical load that reduces wall stress by increasing wall thickness. Chronic hypertrophic remodeling involves cardiac dysfunction that will lead to heart failure and ultimately death. Studies have been carried out on cardiac hypertrophy for years, whereas the mechanisms have not been well defined. Tamarixetin (TAM), a natural flavonoid derivative of quercetin, have been demonstrated possessing anti-oxidative and anti-inflammatory effects on multiple diseases. However, little is known about the function of TAM on the development of cardiac hypertrophy. Here, we found TAM could alleviate pressure-overload-induced cardiac hypertrophy in transverse aortic constriction (TAC) mouse model, assessed by ventricular weight/body weight, lung weight/body weight, echocardiographic parameters, as well as myocyte cross-sectional area and the expression of ANP, BNP and Myh7. In vitro, TAM showed a dose dependent inhibitory effect on phenylephrine-induced hypertrophy in H9c2 cardiomyocytes. Furthermore, TAM reversed cardiac remodeling of stress overloaded heart by suppressing apoptosis and the expression of fibrotic-related genes, reduced oxidative stress and ROS production both in vivo and in vitro. In addition, TAM could negatively modulate TAC-induced nuclear translocation of NFAT and the activation of PI3K/AKT signaling pathways. Therefore, these data indicate for the first time that TAM has a protective effect on experimental cardiac hypertrophy and might be a novel candidate for the treatment of cardiac hypertrophy in clinic.
Keywords: Cardiac hypertrophy; Oxidative stress; Remodeling; Tamarixetin.
J Nat Prod . 2018 Jun 22;81(6):1435-1443.
Abstract
Sepsis is a systemic inflammatory response to pathogenic infection that currently has no specific pharmaceutical interventions. Instead, antibiotics administration is considered the best available option, despite increasing drug resistance. Alternative strategies are therefore urgently required to prevent sepsis and strengthen the host immune system. One such option is Tamarixetin (4'- O-methylquercetin), a naturally occurring flavonoid derivative of quercetin that protects against inflammation. The purpose of this study was to determine whether the anti-inflammatory effects of Tamarixetin protect against the specific inflammatory conditions induced in lipopolysaccharide (LPS) or Escherichia coli K1 models of sepsis. Our study showed that Tamarixetin reduced the secretion of various inflammatory cytokines by dendritic cells after activation with LPS. It also promoted the secretion of the anti-inflammatory cytokine interleukin (IL)-10 and specifically increased the population of IL-10-secreting immune cells in LPS-activated splenocytes. Tamarixetin showed general anti-inflammatory effects in mouse models of bacterial sepsis and decreased bacteria abundance and endotoxin levels. We therefore conclude that Tamarixetin has superior anti-inflammatory properties than quercetin during bacterial sepsis. This effect is associated with an increased population of IL-10-secreting immune cells and suggests that Tamarixetin could serve as a specific pharmaceutical option to prevent bacterial sepsis.
Mol Carcinog. 2014 Dec;53(12):939-50.
Induction of G2/M phase arrest and apoptosis by the flavonoid tamarixetin on human leukemia cells.[Pubmed:
23765509]
Flavonoids are naturally occurring polyphenolic compounds which display a vast array of biological activities.
METHODS AND RESULTS:
In this study, we investigated the effects of Tamarixetin on viability of human tumor cell lines and found that it was cytotoxic against leukemia cells and in particular P-glycoprotein-overexpressing K562/ADR cells. This compound inhibited proliferation in a concentration- and time-dependent manner, induced apoptosis and blocked cell cycle progression at G2 -M phase. This was associated with the accumulation of cyclin B1, Bub1 and p21(Cip1/Waf-1), changes in the phosphorylation status of cyclin B1, Cdk1, Cdc25C and MPM-2, and inhibition of tubulin polymerization. Moreover, cell death was found to be associated with cytochrome c release and cleavage of caspases and of poly(ADP-ribose) polymerase, and completely abrogated by the free-radical scavenger N-acetyl-L-cysteine.
CONCLUSIONS:
The sensitivity of leukemic cells to Tamarixetin suggests that it should be considered for further preclinical and in vivo testing.
Br J Pharmacol. 2006 Apr;147(7):765-71.
Red wine alcohol promotes quercetin absorption and directs its metabolism towards isorhamnetin and tamarixetin in rat intestine in vitro.[Pubmed:
16444288]
Moderate consumption of red wine has been associated with beneficial effects on human health, and this has been attributed to the flavonoid content. Factors that influence the bioavailability of this group of polyphenolic compounds are therefore important.
METHODS AND RESULTS:
Using the rat cannulated everted jejunal sac technique, we have investigated the effect of alcohol on the intestinal absorption of quercetin and its 3-O-glucoside from red wine. Tissue preparations were incubated in whole or dealcoholised red wine, diluted 1 : 1 with Krebs buffer for 20 min at 37 degrees C, after which the mucosa was removed and processed for HPLC analysis. Tissues exposed to red wine had significantly higher amounts of both quercetin (x 3; P < 0.001) and quercetin-3-O-glucoside (x 1.5; P < 0.01) associated with them, compared with sacs incubated in the dealcoholised equivalent.In addition, both Tamarixetin (T) and isorhamnetin (I), in the mucosal tissue from sacs exposed to the whole wine, were significantly elevated approximately two fold (P < 0.05; P < 0.01, respectively). Similar results were obtained when sacs were incubated in Krebs buffer containing a mixture of pure quercetin and quercetin-3-O-glucoside with or without alcohol, and, although effects on the apparent absorption of Q and Q-3-G were not so marked, concentrations of the metabolites quercetin-3-O-glucuronide and I were significantly increased by the presence of alcohol (P < 0.01 and P < 0.001, respectively).
CONCLUSIONS:
It is therefore plausible that the moderate alcohol content of red wine contributes to its beneficial health effects in humans by both increasing the absorption of quercetin and quercetin-3-O-glucoside and by channelling their metabolism towards O-methylation to yield compounds (Tamarixetin and I), which have potential protective effects against cancer and cardiovascular diseases.