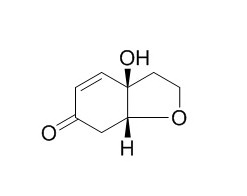
Cleroindicin F
Cleroindicin F shows cytotoxic activity against some human cancer cells. Cleroindicin F and apigenin in the ethyl acetate extract provide scientific support to the traditional use of the leaves of C. splendens for the treatment of infections, wounds and other inflammatory conditions.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Phytother Res.2019, 33(4):1104-1113
Journal of Chromatography A2020, 460942
J Pharm Biomed Anal.2024, 251:116444.
Prev Nutr Food Sci.2024, 29(4):563-571.
Cell Physiol Biochem.2017, 43(4):1425-1435
Foods.2022, 12(1):136.
Phytomedicine Plus2022, 2(1):100207.
Molecules.2019, 25(1):E103
Front Pharmacol.2017, 8:205
J Microbiol Immunol Infect.2021, S1684-1182(21)00142-0.
Related and Featured Products
J Org Chem. 2009 Jun 5;74(11):4104-9.
Enantioselective total synthesis of all of the known chiral cleroindicins (C-F): clarification among optical rotations and assignments.[Pubmed:
19476394]
METHODS AND RESULTS:
Enantioselective syntheses of all of the named chiral members of the Cleroindicin Family (C-F) are reported.
This effort demonstrates the synthetic utility of a 2,4-dihydroxybenzaldehyde as a starting material for natural product synthesis through the use sequential o-quinone methide chemistry and diastereoselective dearomatization. Natural Cleroindicin F was shown to be nearly racemic, and an optically pure synthetic sample of Cleroindicin F was found to racemize under slightly basic conditions.
CONCLUSIONS:
All other natural chiral cleroindicins are shown to be partially racemic.
J Org Chem. 2012 Nov 16;77(22):10294-303.
Tandem sequence of phenol oxidation and intramolecular addition as a method in building heterocycles.[Pubmed:
23110614]
METHODS AND RESULTS:
A tandem phenol oxidation-Michael addition furnishing oxo- and -aza-heterocycles has been developed. Dirhodium caprolactamate [Rh(2)(cap)(4)] catalyzed oxidation by T-HYDRO of phenols with alcohols, ketones, amides, carboxylic acids, and N-Boc protected amines tethered to their 4-position afforded 4-(tert-butylperoxy)cyclohexa-2,5-dienones that undergo Brønsted acid catalyzed intramolecular Michael addition in one-pot to produce oxo- and -aza-heterocycles in moderate to good yields. The scope of the developed methodology includes dipeptides Boc-Tyr-Gly-OEt and Boc-Tyr-Phe-Me and provides a pathway for understanding the possible transformations arising from oxidative stress of tyrosine residues.
CONCLUSIONS:
A novel method of selective cleavage of O-O bond in hindered internal peroxide using TiCl(4) has been discovered in efforts directed to the construction of Cleroindicin F, whose synthesis was completed in 50% yield over just 3 steps from tyrosol using the developed methodology.