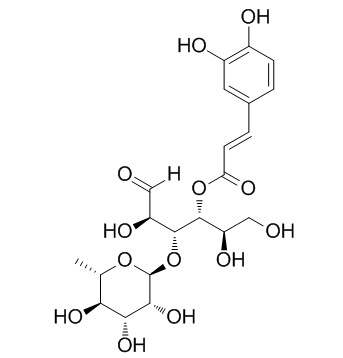
Cistanoside F
Cistanoside F shows vasorelaxant, and antioxidative effects, it shows a strong free radical scavenging activity on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical and xanthine/xanthine oxidase (XOD) generated superoxide anion radical (O2-.).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Agronomy2020, 10(10),1489
Plants (Basel).2023, 12(6):1259.
Appl. Sci.2020, 10(5),1713.
Horticulture Research2023, uhad259
Front Cell Dev Biol.2021, 9:588093.
J Ethnopharmacol.2017, 196:75-83
Nat Prod Sci.2019, 25(3):238
Evid Based Complement Alternat Med.2018, 2018:4580627
Phytomedicine.2024, 125:155350.
Plants (Basel).2024, 13(23):3314.
Related and Featured Products
Bioorg Med Chem. 2006 Nov 15;14(22):7468-75.
Phenylethanoid oligoglycosides and acylated oligosugars with vasorelaxant activity from Cistanche tubulosa.[Pubmed:
16908167 ]
The methanolic extract from the dried stems of Cistanche tubulosa (Schrenk) R. Wight was found to show an inhibitory effect on contractions induced by noradrenaline in isolated rat aortic strips.
METHODS AND RESULTS:
From the extract, new phenylethanoid oligoglycoside constituents, kankanosides F and G, and an acylated oligosugar, kankanose, were isolated together with 14 known compounds. The structures of these new compounds were determined on the basis of their chemical and physicochemical evidence. In addition, principal constituents, kankanoside F, kankanose, echinacoside, acteoside, and Cistanoside F, showed vasorelaxant activity, and several structural requirements for the activity were clarified.
Biol Pharm Bull. 1996 Dec;19(12):1580-5.
Antioxidative effects of phenylethanoids from Cistanche deserticola.[Pubmed:
8996643]
The acetone-H2O (9:1) extract from the stem of Cistanche deserticola showed a strong free radical scavenging activity.
METHODS AND RESULTS:
Nine major phenylethanoid compounds were isolated from this extract. They were identified by NMR as acteoside, isoacteoside, 2'-acetylacteoside, tubuloside B, echinacoside, tubuloside A, syringalide A 3'-alpha-rhamnopyranoside, cistanoside A and Cistanoside F.
All of these compounds showed stronger free radical scavenging activities than alpha-tocopherol on 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical and xanthine/xanthine oxidase (XOD) generated superoxide anion radical (O2-.). Among the nine compounds, isoacteoside and tubuloside B, whose caffeoyl moiety is at 6'-position of the glucose, showed an inhibitory effect on XOD. We further studied the effects of these phenylethanoids on the lipid peroxidation in rat liver microsomes induced by enzymatic and non-enzymatic methods.
METHODS AND RESULTS:
As expected, each of them exhibited significant inhibition on both ascorbic acid/Fe2+ and ADP/NADPH/Fe3+ induced lipid peroxidation in rat liver microsomes, which were more potent than alpha-tocopherol of caffeic acid. The antioxidative effect was found to be potentiated by an increase in the number of phenolic hydroxyl groups in the molecule.
Fitoterapia. 2009 Sep;80(6):358-60.
A new iridoid from Adenosma caeruleum R. Br.[Pubmed:
19442709 ]
METHODS AND RESULTS:
A new iridoid glycoside, adenosmoside, together with five known phenylpropanoids, crenatoside, verbascoside, Cistanoside F, campneoside I, and campneoside II and two known flavonoids, apigenin 7-O-beta-D-glucuronopyranoside and apigenin 7-O-beta-D-glucopyranoside, were isolated from the aerial parts of Adenosma caeruleum R. Br.
CONCLUSIONS:
Their structures were elucidated by spectral evidence.