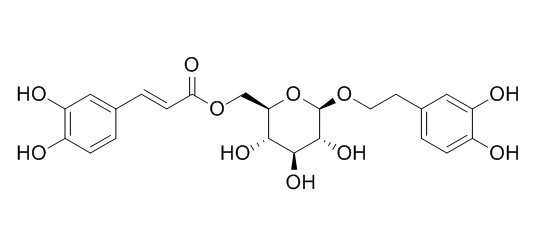
Calceolarioside B
Calceolarioside B has anti-proliferation property.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Cell Biochem Funct.2018, 36(6):303-311
Fundam. Toxicol. Sci.2024, 11(4):197-204
Biomedicines.2022, 10(5):1170
J Microbiol Biotechnol.2024, 35:e2408022.
Plants (Basel).2020, 9(11):1555.
Front Pharmacol.2021, 12:770667.
Planta Medica International2022, 9(01):e108-e115.
Int J Mol Sci.2020, 21(8):2790.
Anal Chim Acta.2021, 1180:338874.
Korean J. Medicinal Crop Sci.2021, 29(6):425-433
Related and Featured Products
Chem Pharm Bull (Tokyo). 2014;62(3):288-93.
Three new lignan glycosides with IL-6 inhibitory activity from Akebia quinata.[Pubmed:
24583784]
METHODS AND RESULTS:
Three new lignan glycosides, akeqintoside A [(7S,8S)-7,8-dihydro-8-hydroxymethyl-7-(4-hydroxy-3-methoxyphenyl)-1'-benzofuranpropanol 2'-O-β-D-glucopyranoside] (1), akeqintoside B [(7R,8R)-7,8-dihydro-8-hydroxymethyl-7-(4-hydroxy-3-methoxyphenyl)-1'-(9'-methoxy-7'-propenyl) benzofuran 2'-O-β-D-glucopyranoside] (2), and akequintoside C [7R*,8R*-dihydroxy-7-(4-hydroxy-3-methoxyphenyl)-glycerol 9-O-β-D-(6'-O-caffeoyl)-glucopyranoside] (3) were isolated from Akebia quinata along with five known compounds, syringin (4), vanilloloside (5), salidroside (6), 3,4-dihydroxyphenylethyl alcohol 8-O-β-D-glucopyranoside (7), and Calceolarioside B (8). The structures of the compounds were identified based on one dimensional (1D)- and 2D-NMR, including (1)H-(1)H correlation spectroscopy (COSY), heteronuclear single quantum coherence (HSQC), heteronuclear multiple bond connectivity (HMBC) and nuclear Overhauser effect spectroscopy (NOESY) spectroscopic analyses.
CONCLUSIONS:
The inhibitory activity of these isolated compounds against interleukin-6 (IL-6) production in tumor necrosis factor-alpha (TNF-α) stimulated MG-63 cells was also examined.
Biomed Res Int . 2017;2017:4273257.
Inhibitory Activities of Stauntonia hexaphylla Leaf Constituents on Rat Lens Aldose Reductase and Formation of Advanced Glycation End Products and Antioxidant[Pubmed:
28326319]
Abstract
Stauntonia hexaphylla (Thunb.) Decne. (Lardizabalaceae) leaves (SHL) have been used traditionally as analgesics, sedatives, diuretics, and so on, in China. To date, no data have been reported on the inhibitory effect of SHL and its constituents on rat lens aldose reductase (RLAR) and advanced glycation end products (AGEs). Therefore, the inhibitory effect of compounds isolated from SHL extract on RLAR and AGEs was investigated to evaluate potential treatments of diabetic complications. The ethyl acetate (EtOAC) fraction of SHL extract showed strong inhibitory activity on RLAR and AGEs; therefore, EtOAc fraction (3.0 g) was subjected to Sephadex LH-20 column chromatography, for further fractionation, with 100% MeOH solvent system to investigate its effect on RLAR and AGEs. Phytochemical investigation of SHL led to the isolation of seven compounds. Among the isolated compounds, chlorogenic acid, Calceolarioside B, luteolin-3'-O-β-D-glucopyranoside, quercetin-3-O-β-D-glucopyranoside, and luteolin-7-O-β-D-glucopyranoside exhibited significant inhibitory activity against RLAR with IC50 in the range of 7.34-23.99 μM. In addition, 3-(3,4-dihydroxyphenyl) propionic acid, neochlorogenic acid, and luteolin-3'-O-β-D-glucopyranoside exhibited the most potent inhibitory activity against formation of AGEs, with an IC50 value of 115.07-184.06 μM, compared to the positive control aminoguanidine (820.44 μM). Based on these findings, SHL dietary supplements could be considered for the prevention and/or treatment of diabetes complication.
Org Biomol Chem. 2014 May 14;12(18):2926-37.
A general synthetic strategy and the anti-proliferation properties on prostate cancer cell lines for natural phenylethanoid glycosides.[Pubmed:
24691797 ]
A general strategy for the synthesis of phenylethanoid glycosides (PhG) including echinacoside 1, acteoside 2, calceolarioside A 3 and Calceolarioside B 4 is reported.
METHODS AND RESULTS:
The strategy features the application of low substrate concentration glycosylation and N-formyl morpholine modulated glycosylation methods for the construction of 1,2-trans β- and α-glycosidic bonds. The reported strategy does not invoke the use of the participatory acyl protecting function, which is incompatible with the ester function present in target PhG compounds.
CONCLUSIONS:
A preliminary study of the anti-proliferation properties of the PhG compounds 1–4 was performed; the acteoside 2 exhibited the best inhibition on the prostatic cancer cell proliferation.