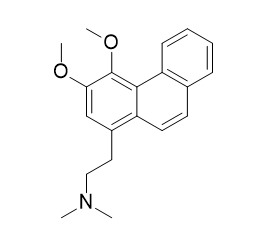
Atherosperminine
Atherosperminine shows cholinesterase inhibitory activity against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). Atherosperminine shows strong anti-plasmodial activity against Plasmodium falciparum, with the IC50 value of 5.80 uM, it also shows antioxidant activity in a DPPH assay with the IC50 value of 54.53 ug/mL. Atherosperminine exerts a non-specific relaxant effect on the trachealis, its major mechanism of action appears to be inhibition of cAMP phosphodiesterase.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Ethnopharmacol.2021, 267:113615.
Journal of Apiculture2019, 34(2):131-136
Molecules.2023, 28(5):2376.
Environ Toxicol.2024, 39(5):2927-2936.
bioRxiv2021, 462065.
J Biosci.2020, 45:46.
Int J Mol Sci.2023, 24(24):17589.
Biochem Pharmacol. 2023, 210:115463.
Biochemistry.2018, 57(40):5886-5896
Korean J Pain.2021, 34(4):405-416.
Related and Featured Products
Molecules. 2013 Jul 8;18(7):8009-17.
Antiplasmodial alkaloids from the bark of Cryptocarya nigra (Lauraceae).[Pubmed:
23884132]
METHODS AND RESULTS:
A dichloromethane extract of the stem bark of Cryptocarya nigra showed strong in vitro inhibition of Plasmodium falciparum growth, with an IC50 value of 2.82 μg/mL. The phytochemical study of this extract has led to the isolation and characterization of four known alkaloids: (+)-N-methylisococlaurine (1), Atherosperminine (2), 2-hydroxyathersperminine (3), and norAtherosperminine (4). Structural elucidation of all alkaloids was accomplished by means of high field 1D- and 2D-NMR, IR, UV and LCMS spectral data. The isolated extract constituents (+)-N-methylisococlaurine (1), Atherosperminine (2) and 2-hydroxy-Atherosperminine (3) showed strong antiplasmodial activity, with IC50 values of 5.40, 5.80 and 0.75 μM, respectively. In addition, (+)-N-methylisocolaurine (1) and Atherosperminine (2) showed high antioxidant activity in a DPPH assay with IC50 values of 29.56 ug/mL and 54.53 ug/mL respectively.
CONCLUSIONS:
Compounds 1 and 2 also both showed high antioxidant activity in the FRAP assay, with percentages of 78.54 and 70.66 respectively and in the metal chelating assay, with IC50 values of 50.08 ug/mL and 42.87 ug/mL, respectively.
Eur J Pharmacol. 1993 Jun 11;237(1):109-16.
The relaxant actions on guinea-pig trachealis of atherosperminine isolated from Fissistigma glaucescens.[Pubmed:
8395388]
The pharmacological activity of Atherosperminine, isolated from Fissistigma glaucescens, was determined in isolated guinea-pig trachealis.
METHODS AND RESULTS:
Atherosperminine (25-100 microM) and theophylline (10-1000 microM) both inhibited the contractile response caused by carbachol, prostaglandin F2 alpha (PGF2 alpha), U46619 (thromboxane A2 analogue), leukotriene C4 (LTC4) and Ca2+ (in the presence of 120 mM KCl) in a concentration-dependent manner. The inhibition was characterized by a rightwards shift of the concentration-response curves with suppression of the maximal contraction. Propranolol (1 microM), glibenclamide (10 microM) and removal of tracheal epithelium did not modify the relaxant action of Atherosperminine. Atherosperminine (25 and 50 microM) caused a 2.4- and 5.0-fold, respectively, potentiation of the action of forskolin to cause tracheal relaxation but did not potentiate the action of sodium nitroprusside or cromakalim. Atherosperminine (50 microM) potentiated the action of forskolin to increase tissue cAMP content and, in higher concentrations (100 and 250 microM), itself increased tissue cAMP but not cGMP content. Atherosperminine markedly inhibited cAMP phosphodiesterase but not cGMP phosphodiesterase in homogenates of guinea-pig trachealis.
CONCLUSIONS:
It is concluded that Atherosperminine exerts a non-specific relaxant effect on the trachealis. Its major mechanism of action appears to be inhibition of cAMP phosphodiesterase, perhaps with a minor effect on cGMP phosphodiesterase at higher concentrations.
Bioorg Med Chem. 2016 Sep 15;24(18):4464-4469.
Cholinesterase inhibitory activity of isoquinoline alkaloids from three Cryptocarya species (Lauraceae).[Pubmed:
27492195 ]
Alzheimer's disease is the most common form of dementia among older adults. Acetylcholinesterase and butyrylcholinesterase are two enzymes involved in the breaking down of the neurotransmitter acetylcholine. Inhibitors for these enzymes have potential to prolong the availability of acetylcholine. Hence, the search for such inhibitors especially from natural products is needed in developing potential drugs for Alzheimer's disease.
METHODS AND RESULTS:
The present study investigates the cholinesterase inhibitory activity of compounds isolated from three Cryptocarya species towards acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). Nine alkaloids were isolated; (+)-nornantenine 1, (-)-desmethylsecoantofine 2, (+)-oridine 3, (+)-laurotetanine 4 from the leaves of Cryptocarya densiflora BI., Atherosperminine 5, (+)-N-methylisococlaurine 6, (+)-N-methyllaurotetanine 7 from the bark of Cryptocarya infectoria Miq., 2-methoxyAtherosperminine 8 and (+)-reticuline 9 from the bark of Cryptocarya griffithiana Wight. In general, most of the alkaloids showed higher inhibition towards BChE as compared to AChE. The phenanthrene type alkaloid; 2-methoxyAtherosperminine 8, exhibited the most potent inhibition against BChE with IC50 value of 3.95μM. Analysis of the Lineweaver-Burk (LB) plot of BChE activity over a range of substrate concentration suggested that 2-methoxyAtherosperminine 8 exhibited mixed-mode inhibition with an inhibition constant (Ki) of 6.72μM.
CONCLUSIONS:
Molecular docking studies revealed that 2-methoxyAtherosperminine 8 docked well at the choline binding site and catalytic triad of hBChE (butyrylcholinesterase from Homo sapiens); hydrogen bonding with Tyr 128 and His 438 residues respectively.