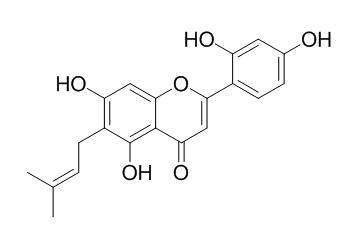
Artocarpesin
Artocarpesin has anti-inflammatory effects, it can suppress the LPS-induced production of nitric oxide (NO) and prostaglandin E 2 (PGE 2) through the down-regulation of inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) protein expressions. Artocarpesin displays cytotoxic effects at various extent on all the 9 tested cancer cell lines with IC50 values respectively below 106 uM. Artocarpesin has interesting antimicrobial potency, and shows tyrosinase inhibitory activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Food Bioscience2023, 59:103903
Pharm Biol.2021, 59(1):134-145.
J Biol Chem.2021, 297(6):101362.
University of Limpopo2016, 1777
J Agric Food Chem.2024, 72(40):22237-22249.
China Pharmacy2015, 26(27)
University of Stuttgart2021, 11682.
FASEB J.2019, 33(2):2026-2036
Planta Med.2019, 85(4):347-355
Korean J Dent Mater.2018, 45(2):139-146
Related and Featured Products
Phytomedicine. 2015 Nov 15;22(12):1096-102.
Cytotoxicity of three naturally occurring flavonoid derived compounds (artocarpesin, cycloartocarpesin and isobavachalcone) towards multi-factorial drug-resistant cancer cells.[Pubmed:
26547532]
Cancer remains an aggressive deadly disease, if drug resistance develops. This problem is aggravated by the fact that multiple rather than single mechanisms are involved in resistance and that multidrug resistance (MDR) phenomena cause inefficacy of many clinical established anticancer drugs. We are seeking for novel cytotoxic phytochemicals to combat drug-resistant tumour cells.
METHODS AND RESULTS:
In the present study, we investigated the cytotoxicity of three naturally occurring flavonoids including two flavones Artocarpesin (1) and cycloArtocarpesin (2) and one chalcone, isobavachalcone (3) against 9 drug-sensitive and MDR cancer cell lines. The resazurin reduction assay was used to evaluate the cytotoxicity of these compounds, whilst caspase-Glo assay was used to detect caspase activation. Cell cycle, mitochondrial membrane potential (MMP) and levels of reactive oxygen species (ROS) were all analysed via flow cytometry. Flavones 1 and 2 as well as chalcone 3 displayed cytotoxic effects at various extent on all the 9 tested cancer cell lines with IC50 values respectively below 106 μM, 50 μM and 25 μM. The IC50 values for the three investigational flavonoids ranged from 23.95 μM (towards hepatocarcinoma HepG2 cells) to 105 μM [towards colon carcinoma HCT116 (p53(-/-)) cells] for 1, from 15.51 μM (towards leukemia CCRF-CEM cells) to 49.83 μM [towards glioblastoma U87MG.ΔEGFR cells] for 2 and from 2.30 μM (towards CCRF-CEM cells) to 23.80 μM [towards colon carcinoma HCT116 (p53(+/+)) cells] for 3 and from 0.20 μM (towards CCRF-CEM cells) to 195.12 μM (towards leukemia CEM/ADR5000 cells) for doxorubicin. Compounds 2 and 3 induced apoptosis in CCRF-CEM leukemia cells, mediated by caspase activation and the disruption of MMP.
CONCLUSIONS:
The three tested flavonoids and mostly chalcone 3 are potential cytotoxic natural products that deserve more investigations to develop novel antineoplastic drugs against multifactorial drug-resistant cancers.
Nat Prod Commun. 2011 Sep;6(9):1397-402.
Artocarpus plants as a potential source of skin whitening agents.[Pubmed:
21941923]
Artocarpus plants have been a focus of constant attention due to the potential for skin whitening agents. In the in vitro experiment, compounds from the Artocarpus plants, such as artocarpanone, norartocarpetin, Artocarpesin, artogomezianol, andalasin, artocarbene, and chlorophorin showed tyrosinase inhibitory activity. Structure-activity investigations revealed that the 4-substituted resorcinol moiety in these compounds was responsible for their potent inhibitory activities on tyrosinase.
METHODS AND RESULTS:
In the in vitro assay, using B16 melanoma cells, the prenylated polyphenols isolated from Artocarpus plants, such as artocarpin, cudraflavone C, 6-prenylapigenin, kuwanon C, norartocarpin, albanin A, cudraflavone B, and brosimone I showed potent inhibitory activity on melanin formation. Structure-activity investigations revealed that the introduction of an isoprenoid moiety to a non-isoprenoid-substituted polyphenol enhanced the inhibitory activity of melanin production in B16 melanoma cells. In the in vivo investigation, the extract of the wood of Artocarpus incisus and a representative isolated compound from it, artocarpin had a lightening effect on the skin of guinea pigs' backs. Other in vivo experiments using human volunteers have shown that water extract of Artocarpus lakoocha reduced the melanin formation in the skin of volunteers.
CONCLUSIONS:
These results indicate that the extracts of Artocarpus plants are potential sources for skin whitening agents.
J Ethnopharmacol. 2009 Jul 30;124(3):551-5.
Antimicrobial activity of the methanolic extract and compounds from Morus mesozygia stem bark.[Pubmed:
19450674]
This study was aimed at investigating the antimicrobial activity of the methanolic extract (MMB) and compounds isolated from the stem bark of Morus mesozygia, namely 3beta-acetoxyurs-12-en-11-one (1), moracin Q (2), moracin T (3), Artocarpesin (4), cycloArtocarpesin (5), moracin R (6), moracin U (8), moracin C (9), and moracin M (10).
METHODS AND RESULTS:
The liquid microdilution assay was used in the determination of the minimal inhibitory concentration (MIC) and the minimal microbicidal concentration (MMC), against nine bacterial and two fungal species. The results of the MIC determination showed that the compounds 3, 4, 8 and 9 were able to prevent the growth of all tested microbial species. All other samples showed selective activities. Their inhibitory effects were noted on 90.9% studied organisms for the crude extract, 81.8% for compound 6, 72.7% for compound 10, 63.6% for compound 1, 54.5% for compound 5, and 45.5% for compound 2. The lowest MIC value of 39 microg/ml was obtained with the crude extract against Escherichia coli. The corresponding value for compounds (5 microg/ml) was registered with compound 9 on Shigella dysenteriae and compound 3 on E. coli, S. dysenteriae, Pseudomonas aeruginosa, Salmonella typhi and Bacillus cereus. The lowest MIC value (39 microg/ml) observed with the crude extract (on E. coli) was only eightfold greater than that of gentamycin used as reference antibiotic (RA) while the corresponding value (5 microg/ml) recorded with compounds 3 and 9 was equal to that of RA on the corresponding microorganisms.
CONCLUSIONS:
The obtained results highlighted the interesting antimicrobial potency of M. mesozygia as well as that of the studied compounds, and provided scientific basis for the traditional use of this species.
J Agric Food Chem. 2008 Jun 25;56(12):4463-8.
Anti-inflammatory effects of phenolic compounds isolated from the fruits of Artocarpus heterophyllus.[Pubmed:
18500810]
Artocarpus heterophyllus Lam is a large evergreen tree cultivated throughout Southeast Asia for its fruits. Its leaves and roots have been used for medicinal purposes. The aim of this work was to study the in vitro anti-inflammatory effects of phenolic compounds isolated from the ethyl acetate extracts of the fruits of Artocarpus heterophyllus.
METHODS AND RESULTS:
Three phenolic compounds were characterized as Artocarpesin [5,7,2',4'-tetrahydroxy-6-(3-methylbut-3-enyl) flavone] ( 1), norartocarpetin (5,7,2',4'-tetrahydroxyflavone) ( 2), and oxyresveratrol [ trans-2,4,3',5'-tetrahydroxystilbene] ( 3) by spectroscopic methods and through comparison with data reported in the literatures. The anti-inflammatory effects of the isolated compounds ( 1- 3) were evaluated by determining their inhibitory effects on the production of proinflammatory mediators in lipopolysaccharide (LPS)-activated RAW 264.7 murine macrophage cells. These three compounds exhibited potent anti-inflammatory activity.
CONCLUSIONS:
The results indicated that Artocarpesin ( 1) suppressed the LPS-induced production of nitric oxide (NO) and prostaglandin E 2 (PGE 2) through the down-regulation of inducible nitric oxide synthase (iNOS) and cyclooxygenase 2 (COX-2) protein expressions. Thus, Artocarpesin ( 1) may provide a potential therapeutic approach for inflammation-associated disorders.
Mol Nutr Food Res. 2008 Dec;52(12):1530-8.
Isolation of tyrosinase inhibitors from Artocarpus heterophyllus and use of its extract as antibrowning agent.[Pubmed:
18683821 ]
A new furanoflavone, 7-(2,4-dihydroxyphenyl)-4-hydroxy-2-(2-hydroxy propan-2-yl)-2, 3-dihydrofuro(3, 2-g)chromen-5-one (artocarpfuranol, 1), together with 14 known compounds, dihydromorin (2), steppogenin (3), norartocarpetin (4), artocarpanone (5), Artocarpesin (6), artocarpin (7), cycloartocarpin (8), cycloArtocarpesin (9), artocarpetin (10), brosimone I (11), cudraflavone B (12), carpachromene (13), isoArtocarpesin (14), and cyanomaclurin (15) were isolated from the wood of Artocarpus heterophyllus.
METHODS AND RESULTS:
Their structures were identified by interpretation of MS,( 1)H-NMR,( 13)C-NMR, HMQC, and HMBC spectroscopic data. Among them, compounds 1-6 and 14 showed strong mushroom tyrosinase inhibitory activity with IC(50) values lower than 50 microM, more potent than kojic acid (IC(50) = 71.6 microM), a well-known tyrosinase inhibitor. In addition, extract of A. heterophyllus was evaluated for its antibrowning effect on fresh-cut apple slices. It was discovered that fresh-cut apple slices treated by dipping in solution of 0.03 or 0.05% of A. heterophyllus extract with 0.5% ascorbic acid did not undergo any substantial browning reaction after storage at room temperature for 24 h. The antibrowning effect was significantly better than samples treated with the extract (0.03 or 0.05%) or ascorbic acid (0.5%) alone.
CONCLUSIONS:
The results provide preliminary evidence supporting the potential of this natural extract as antibrowning agent in food systems.