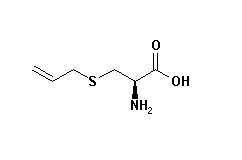
Allylcysteine
S-allylcysteine has antioxidant and chemopreventive effects. S-allylcysteine is protective in myocardial infarction via an H 2S-related pathway, it mediates cardioprotection in an acute myocardial infarction rat model via a hydrogen sulfide-mediated pathway, it also ameliorates doxorubicin toxicity in the heart and liver in mice. S-Allylcysteine prevents amyloid-β peptide-induced oxidative stress in rat hippocampus and ameliorates learning deficits
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Phytochemistry Letters2017, 449-455
Int J Biol Macromol.2020, 161:1230-1239.
Agronomy2023, 13(6), 1435.
Pharmacia2022, 69(3): 883-890.
Antioxidants (Basel).2021, 10(1):112.
Pharmacol Rep.2020, 72(2):472-480.
Plant Cell Tiss Org2017, 479-486
Nutrients.2020, 12(12):3638.
Phytomedicine.2019, 62:152962
Life (Basel).2023, 13(2):457.
Related and Featured Products
Free radical biology & medicine, 2003, 35(3):317-324.
Antioxidant S-allylcysteine prevents gentamicin-induced oxidative stress and renal damage.[Reference:
WebLink]
Acute renal failure (ARF) is a major complication of gentamicin (GM) treatment, which is effective against gram-negative infections. Since experimental evidence suggests a role of reactive oxygen species (ROS) in GM-induced ARF, in this work we studied the effect of a garlic-derived compound, S-Allylcysteine (SAC), which is a free radical scavenger, on GM-induced nephrotoxicity.
METHODS AND RESULTS:
In rats treated with GM (70 mg/kg/12 h/4 days/s.c.), ARF was evident by the: (i) decrease in creatinine clearance and increase in blood urea nitrogen, (ii) decrease in blood glutathione peroxidase (GPx) activity and increase in urinary excretion of N-acetyl-β-D-glucosaminidase and total protein, and (iii) necrosis of proximal tubular cells. These alterations were prevented by SAC treatment (250 mg/kg/i.p. 24 h before the first dose of GM and 125 mg/kg/12 h/4 days along GM-treatment). Furthermore, SAC prevented the GM-induced oxidative stress (protein carbonyl groups) and the decrease in manganese superoxide dismutase (Mn-SOD), GPx, and glutathione reductase (GR) activities in renal cortex.
CONCLUSIONS:
In conclusion, SAC ameliorates the GM-induced ARF by a mechanism related, at least in part, to its ability to decrease oxidative stress and to preserve antioxidant enzymes activity in renal cortex.
Carcinogenesis, 1996, 17(5):1041-1044.
Chemopreventive effect of S-allylcysteine and its relationship to the detoxification enzyme glutathione S-transferase.[Reference:
WebLink]
Sulfur-containing substances derived from garlic and onion have been shown to prevent experimental carcinogenesis. One of the hypotheses explaining the mechanisms of the chemopreventive activity of these substances is that they activate detoxification systems such as glutathione S-transferase (GST). In this study the effects of S-Allylcysteine (SAC), a water-soluble organosulfur compound derived from garlic, on GST activities in the liver, small intestine and colon were investigated. Additionally, we examined SAC for chemopreventive effects on aberrant crypt foci, which are the most likely precursors of colon cancers.
METHODS AND RESULTS:
In the rat colonic aberrant crypt assay administration of SAC during the initiation period decreased the number of aberrant crypt foci by 33 and 54% in groups given 40 or 80% maximum tolerated dose (MTD) of SAC respectively. The number of aberrant crypt foci, however, was not changed when SAC was given during the promotion period. GST activity in the liver was increased significantly by 41% 12 h after a single oral administration of 3.5 mmol/kg SAC and this elevated GST level was maintained over a 72 h period. GST levels were increased significantly by the administration of SAC (1.8 mmol/kg/ day for 3 days) not only in the liver but also in the proximal and middle small bowel. Isozyme levels of GST after administration of SAC were also determined using Western blotting. Hepatic GST-alpha and GST-mu were significantly increased by 35 and 42% respectively after oral administration of SAC. GST-pi levels were lower than the detection limit (130 ng/mg/protein) in both the control and SAC-treated groups.
CONCLUSIONS:
These results strongly support the previous working hypothesis that SAC exhibits chemopreventive activity by exerting specific effects on carcinogen detoxification systems.
European journal of pharmacology, 2004, 489(3):197-202.
S-Allylcysteine prevents amyloid-β peptide-induced oxidative stress in rat hippocampus and ameliorates learning deficits.[Reference:
WebLink]
METHODS AND RESULTS:
The effects of S-Allylcysteine on oxidative damage and spatial learning and memory deficits produced by an intrahippocampal injection of amyloid-beta peptide 25-35 (Abeta(25-35)) in rats were investigated. The formation of reactive oxygen species, lipid peroxidation and the activities of the antioxidant enzymes superoxide dismutase and glutathione peroxidase were all measured in hippocampus 120 min after Abeta(25-35) injection (1 microl of 100 microM solution), while learning and memory skills were evaluated 2 and 35 days after the infusion of Abeta(25-35) to rats, respectively. Abeta(25-35) increased both reactive oxygen species and lipid peroxidation, whereas pretreatment with S-Allylcysteine (300 mg/kg, i.p.) 30 min before peptide injection decreased both of these markers. In addition, Abeta(25-35)-induced incorrect learning responses were prevented in most of trials by S-Allylcysteine. In contrast, enzyme activities were found unchanged in all groups tested.
CONCLUSIONS:
Findings of this work: (i) support the participation of reactive oxygen species in Abeta(25-35)-induced hippocampal toxicity and learning deficits; and (ii) suggest that the protective effects of S-Allylcysteine were related to its ability to scavenge reactive oxygen species.
Planta Medica, 01 Mar 2000, 66(2):148-151.
S-allylcysteine ameliorates doxorubicin toxicity in the heart and liver in mice.[Reference:
WebLink]
Doxorubicin, a potent anticancer drug, is effective against a wide range of human neoplasms. However, the clinical uses of doxorubicin have been limited due to its serious cardiotoxic effects, which are likely the result of generation of free radicals and lipid peroxidation. S-Allylcysteine (SAC), an organosulfur compound purified from garlic, has been reported to have antioxidant and radical scavenging effects. Thus, we examined the effect of SAC on doxorubicin toxicity in mice.
METHODS AND RESULTS:
Severe doxorubicin toxicity was induced in mice by a single intraperitoneal injection (15 mg/kg body weight). SAC (30 mg/kg) was injected intraperitoneally daily for 5 days, starting two days prior to the administration of doxorubicin. Body weight was measured every alternate day. A measurement of serum creatine phosphokinase (CPK) and a histopathological analysis of the heart and liver was performed 6 days after the administration of doxorubicin. Death of any of the animals was recorded during the observation period. Doxorubicin injection induced a mortality rate of 58%, with SAC treatment reducing the doxorubicin-induced mortality rate to 30%. The severe body weight loss caused by doxorubicin (13%) was also significantly attenuated by SAC treatment (9%). Although an elevation of the level of serum CPK was observed following doxorubicin injection (5472 +/- 570 i.u./L), treatment with SAC significantly reduced the level of CPK (1923 +/- 635 i.u./L). Histological analysis demonstrated that heart and liver damage was significantly less severe in SAC treated mice than in mice receiving only doxorubicin.
CONCLUSIONS:
These results suggest that SAC research may ultimately lead to a resolution of the adverse effects of doxorubicin treatment in cancer chemotherapy.
American journal of physiology heart & circulatory physiology, 2007, 293(5):H2693.
S-Allylcysteine mediates cardioprotection in an acute myocardial infarction rat model via a hydrogen sulfide-mediated pathway.[Reference:
WebLink]
S-Allylcysteine (SAC) is an organosulfur-containing compound derived from garlic. Studies have shown that garlic is beneficial in the treatment of cardiovascular diseases. This study aims to elucidate if SAC is responsible for this cardioprotection using acute myocardial infarction (AMI) rat models. In addition, we hypothesized that SAC may mediate cardioprotection via a hydrogen sulfide (H2S)-related pathway.
METHODS AND RESULTS:
Rats were pretreated with saline, SAC (50 mg·kg-1·day-1), SAC + propagylglycine (PAG; 50 mg + mg·kg-1·day-1) or PAG (10 mg·kg-1·day-1) for 7 days before AMI induction and killed 48 h after. Our results showed that SAC significantly lowered mortality (12.5% vs. 33.3%, P < 0.05) and reduced infarct size. SAC + PAG- and PAG-treated rats had larger infarct sizes than controls (60.9 ± 0.01 and 62.0 ± 0.03%, respectively, vs. 50.0 ± 0.03%; P < 0.05). Pretreatment with SAC did not affect BP, but BP was significantly elevated in SAC + PAG and PAG-treated groups (P < 0.05). In addition, plasma H2S levels and left ventricular cystathionine-γ-lyase (CSE) activities were analyzed to investigate the involvement of H2S. CSE is the enzyme responsible for H2S production in the heart. SAC increased left ventricular CSE activity in AMI rats (2.75 ± 0.34 vs. 1.23 ± 0.16 μmol·g protein-1·h-1; P < 0.01). SAC + PAG-treated rats had significantly lower CSE activity compared with the SAC-treated group (1.22 ± 0.27 vs. 2.75 ± 0.34 μmol·g protein-1·h-1; P < 0.05). Similarly, SAC-treated rats had higher plasma H2S concentration compared with controls and the SAC + PAG-treated group. Protein expression studies revealed that SAC upregulated CSE expression (1.1-fold of control; P < 0.05), whereas SAC + PAG and PAG downregulated its expression (0.88-fold of control in both groups; P < 0.005).
CONCLUSIONS:
In conclusion, our study provides novel evidence that SAC is protective in myocardial infarction via an H 2S-related pathway.