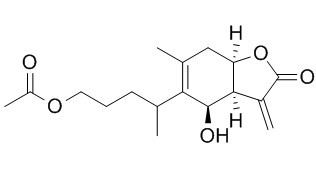
1-O-Acetyl britannilactone
1-O-Acetyl britannilactone shows antifungal, and anti-cancer activities, it suppresses angiogenesis and lung cancer cell growth possibly via regulating the VEGFR-Src-FAK signaling. 1-O-Acetyl britannilactone may be one anti-inflammatory drug which inhibits the expression of COX-2 gene by blocking NF-κB activation and thus suppresses the inflammatory response to LPS in vascular smooth muscle cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Pharmaceuticals (Basel).2024, 17(3):341.
Antioxidants (Basel).2021, 10(9):1435.
Phytochemistry.2021, 181:112539.
Research J. Pharm. and Tech.2020, 13(7):3059-3064.
Food Sci Biotechnol.2024, 34(3):611-620.
Antibiotics.2022, 11(4), 510.
Plants (Basel).2021, 10(6):1192.
Sci Rep.2015, 5:13194
Clin Transl Med.2021, 11(5):e392.
Molecules.2022, 27(19):6681.
Related and Featured Products
Acta Phytophylacica Sinica, 2008 , 35 (6) :551-6.
Separation and analysis of 1-O-acetylbritannilactone from Inula japonica and determination of antifungal activity[Reference:
WebLink]
In order to clarify the main antifungal compound of Inula japonica,the chloroform extraction of crude extract from I.japonica flowers was separated and compound 1-O-Acetyl britannilactone (ABL) was obtained.
METHODS AND RESULTS:
The content of the compound ABL in flowers crude extract was tested with high performance liquid chromatography.The inhibitory effects of compound ABL against Sphaerotheca fuliginea,Pseudoperonospora cubensis,Botrytis cinerea,Alternaria solani and Phytophthora infestans were tested.The chemical structure of ABL reference substance was elucidated by using ultraviolet spectrometry(UV),infrared spectroscopy(IR),nuclear magnetic resonance(NMR).The purity of ABL reference substance was 99.5%,which satisfies the need of reference substances of traditional Chinese medicines.A method of high performance liquid chromatography was used for the determination of the content of ABL in crude extract from I.japonica flowers(it's 2.45%),then the content of ABL in I.japonica was calculated(it's about 0.25%).
CONCLUSIONS:
Inhibitory activities of compound ABL against the five phytopathogenic fungi were different,which against the cystospore germination of P.infestans was significant,the inhibition rate was 100% at the concentration 0.1 mg/mL.
Chinese Journal of Cell Biology, 2007 , 29 (2) :267-71.
1-o-acetylbritannilactone Inhibits Inflammatory Response by Suppressing NF-κB Activation[Reference:
WebLink]
Immunocytochemistry and Western blot analysis were adopted to measure the nuclear translo-cation of NF-κB p65 and the expression of IκB-α, cyclo-oxygenase-2 (COX-2).
METHODS AND RESULTS:
Electrophoretic mobility shift assay (EMSA) was performed to detect DNA-binding activity of NF-κB in vascular smooth muscle cells (VSMC) pre-treated with ABL. Western blot and immunocytochemistry analysis showed that lipopolysaccharide (LPS) treatment resulted in increasing nuclear translocation of NF-κB p65, and declining levels of IκB-α in VSMC. However, 1-O-Acetyl britannilactone (ABL) pretreatment inhibited the nuclear translocation of p65 and degradation of IκB-α in-duced by LPS, and the inhibitory effect of ABL was concentration-dependent. LPS increased the binding of nuclear extracts from VSMC induced by LPS to double strands oligonucleotide probe containing NF-κB binding site using EMSA. The shift bands were abolished when a 100-fold excess of unlabeled NF-κB oligonucleotide probe was included. Pretreatment with ABL significantly reduced the nuclear level of NF-κB and declined the binding activity of nuclear extracts with DNA probe induced by LPS. Furthermore, ABL consequentially inhibited the expression of NF-κB-dependent COX-2 gene induced by LPS.
CONCLUSIONS:
These results suggest that ABL may be one anti-inflammatory drug which inhibits the expression of COX-2 gene by blocking NF-κB activation and thus suppresses the inflammatory response to LPS in VSMC.
Biochem Biophys Res Commun. 2015 Aug 21;464(2):422-7.
1-o-acetylbritannilactone (ABL) inhibits angiogenesis and lung cancer cell growth through regulating VEGF-Src-FAK signaling.[Pubmed:
26102035]
The search for safe, effective and affordable therapeutics against non-small cell lung cancer (NSCLC) and other lung cancers is important.
METHODS AND RESULTS:
Here we explored the potential effect of1-O-Acetyl britannilactone(ABL), a novel extract from Inula britannica-F, on angiogenesis and lung cancer cell growth. We demonstrated that ABL dose-dependently inhibited vascular endothelial growth factor (VEGF)-induced proliferation, migration, and capillary structure formation of cultured human umbilical vascular endothelial cells (HUVECs). In vivo, ABL administration suppressed VEGF-induced new vasculature formation in Matrigel plugs. For the mechanism investigations, we found that ABL largely inhibited VEGF-mediated activation of Src kinase and focal adhesion kinase (FAK) in HUVECs. Furthermore, treatment of A549 NSCLC cells with ABL resulted in cell growth inhibition and Src-FAK in-activation. Significantly, administration of a single dose of ABL (12 mg/kg/day) remarkably suppressed growth of A549 xenografts in nude mice. In vivo microvessels formation and Src activation were also significantly inhibited in ABL-treated xenograft tumors.
CONCLUSIONS:
Taken together, our findings suggest that ABL suppresses angiogenesis and lung cancer cell growth possibly via regulating the VEGFR-Src-FAK signaling.
Se Pu. 2005 Nov;23(6):573-6.
Preparation and determination of 1-O-acetylbritannilactone in Inula Britannica L.[Pubmed:
16498983]
To prepare reference substance for quality control of Inula Britannica L., 1-O-Acetyl britannilactone was extracted and separated from chloroform extraction of Inula Britannica L.
METHODS AND RESULTS:
Chemical structure of the 1-O-acetylbritannilactone product was elucidated by ultraviolet spectrometry (UV), infrared spectroscopy (IR), nuclear magnetic resonance (NMR) and mass spectrometry (MS), and the results were in agreement with the reference. The purity of the 1-O-Acetyl britannilactone product was 99.5%, which satisfies the need of reference substances of traditional Chinese medicines. A method of high performance liquid chromatography-evaporative light scattering detection (HPLC-ELSD) is described for the determination of 1-O-Acetyl britannilactone in Inula Britannica L. The chromatographic conditions include Hypersil ODS-2 column, a mixture of methanol-water (52: 48, v/v) with the flow rate of 1.0 mL/min used as mobile phase. The temperature of drift tube of the ELSD was 90 degrees C. Flow rate of carrier gas was 2.5 L/min. Linear range of 1-O-Acetyl britannilactone was 1.37 - 8.21 microLg (r = 0.999 8). The average recovery of 1-O-acetylbritannilactone was 100.2% with the relative standard deviation (RSD) of 1.3% (n = 6).
CONCLUSIONS:
The method is simple, accurate, time saving and reproducible.