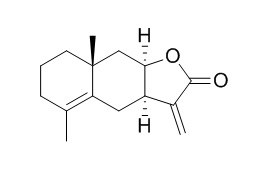
Alloalantolactone
Alloalantolactone shows activity against M. tuberculosis (32 micrograms/ml).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Foods.2024, 13(11):1739.
J Vet Sci.2020, 21(3):e39.
Evid Based Complement Alternat Med.2021, 2021:8707280.
Plant Physiol.2024, 194(4):2580-2599.
Applied Biological Chemistry2022, 65(77).
Processes2022, 10(10), 2008.
Toxicol In Vitro.2018, 52:94-105
Sci Rep.2025, 15(1):29590.
JEJU National University2022, 10478.
Int J Mol Sci.2023, 24(6):5769.
Related and Featured Products
Planta Med. 1999 May;65(4):351-5.
Antimycobacterial eudesmanolides from Inula helenium and Rudbeckia subtomentosa.[Pubmed:
10364842]
In a bioassay guided search for antimycobacterial compounds from higher plants, the root extracts of Elecampane (Inula helenium L.; Asteraceae) and Sweet Coneflower (Rudbeckia subtomentosa Pursh.; Asteraceae) were chemically investigated for their active constituents.
METHODS AND RESULTS:
Chromatographic fractions of root extracts of l. helenium, which exhibited significant activity against Mycobacterium tuberculosis, provided the known eudesmanolides alantolactone, isoalantolactone, and 11 alpha H, 13-dihydroisoalantolactone. Peracid epoxidation of alantolactone and isoalantolactone provided 5 alpha-epoxyalantolactone and 4(15) alpha-epoxyisoalantolactone, respectively and oxidation of alantolactone with OsO4 gave 11,13-dihydroxyalantolactone. Active fractions from R subtomentosa contained the known Alloalantolactone and 3-oxoAlloalantolactone. The structures of the above compounds were established by spectroscopic methods including 1D and 2D NMR techniques as well as spectral comparison with previously reported data. The molecular structure of 5 alpha-epoxyalantolactone was determined by single crystal X-ray diffraction. Eleven natural and semisynthetic eudesmanolides were tested in a radiorespirometric bioassay for activity against M. tuberculosis. 5 alpha-Epoxyalantolactone and encelin from Montanoa speciosa showed minimum inhibitory concentrations (MICs) of 8 and 16 micrograms ml-1, respectively. Alantolactone, isoalantolactone and its 4 alpha, 15-epoxide, 1,2-dehydro-3-epi-isotelekin and Alloalantolactone gave MICs of 32 micrograms ml-1.
CONCLUSIONS:
All other compounds showed MIC values of 128 micrograms ml-1 or higher.