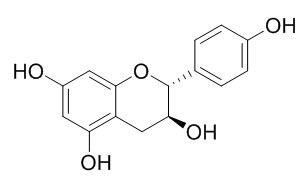
(+)-Afzelechin
(+)-Afzelechin has a neuroprotective effect on the glutamate-induced neurotoxicity in HT22 cells, it has ABTS cation radical scavenging effects with IC50 values of 23.7 microM; it also shows moderate to strong radical scavenging properties against diphenylpicrylhydrazyl radical (DPPH) and improves the reduced glutathione levels in rat pancreatic homogenate.(+)-Afzelechin has inhibitory compound of alpha-glucosidase activity, the ID(50) (50% inhibition dose) value is 0.13 mM.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Heliyon.2024, 10(7):e28755.
PLoS One.2021, 16(9):e0257243.
World J.Traditional Chinese Med.2024, 10(3):370-382
Phytomedicine Plus2022, 2(1):100207.
Molecules.2024, 29(24):5983.
Food Chemistry: X2023, 101032.
Life Sci.2019, 216:259-270
J of l. Chroma.&Related Tech2020, 43(11-12):414-423.
Talanta.2023, 262:124690.
Anesth Pain Med (Seoul).2020, 15(4):478-485.
Related and Featured Products
Phytochemistry. 2008 Jan;69(2):451-6.
Antioxidant aryl-prenylcoumarin, flavan-3-ols and flavonoids from Eysenhardtia subcoriacea.[Pubmed:
17892888]
METHODS AND RESULTS:
Antioxidant activity (AOA) assay-guided chemical analysis, using a rat pancreas homogenate model, of aerial parts from Eysenhardtia subcoriacea, led to isolation of the new compound subcoriacin (3-(2'-hydroxy-4',5'-methylendioxyphenyl)-6-(3''-hydroxymethyl-4''-hydroxybut-2''-enyl)-7-hydroxycoumarin) together with the known substances: (+)-catechin, (-)-epicatechin, (+)-Afzelechin, eriodictyol, (+)-catechin 3-O-beta-D-galactopyranoside and quercetin 3-O-beta-D-galactopyranoside as bioactive constituents. The structure of the compound was determined from 1D and 2D NMR spectroscopic analyses. Additional known constituents were characterized.
CONCLUSIONS:
The bioactive compounds showed also moderate to strong radical scavenging properties against diphenylpicrylhydrazyl radical (DPPH). In addition, subcoriacin, (+)-catechin, (-)-epicatechin and (+)-Afzelechin improved the reduced glutathione levels in rat pancreatic homogenate.
Arch Pharm Res. 2005 Jul;28(7):804-9.
Neuroprotective and free radical scavenging activities of phenolic compounds from Hovenia dulcis.[Pubmed:
16114495]
METHODS AND RESULTS:
The EtOAc-soluble fraction from a methanolic extract of Hovenia dulcis Thunb. exhibited neuroprotective activity against glutamate-induced neurotoxicity in mouse hippocampal HT22 cells.
The neuroprotective activity-guided isolation resulted in 8 phenolic compounds (1-8), such as vanillic acid (1), ferulic acid (2), 3,5-dihydroxystilbene (3), (+)-aromadendrin (4), methyl vanillate (5), (-)-catechin (6), 2,3,4-trihydrobenzoic acid (7), and (+)-Afzelechin (8). Among these, compounds 6 and 8 had a neuroprotective effect on the glutamate-induced neurotoxicity in HT22 cells. Furthermore, compound 6 had a DPPH free radical scavenging effect with an IC50 value of 57.7 microM, and a superoxide anion radical scavenging effect with an IC50 value of 8.0 microM. Both compounds 6 and 8 had ABTS cation radical scavenging effects with IC50 values of 7.8 microM and 23.7 microM, respectively.
CONCLUSIONS:
These results suggest that compounds 6 and 8 could be neuroprotectants owing to their free radical scavenging activities.
J Oleo Sci. 2008;57(8):431-5.
Alpha-glucosidase inhibitor from Bergenia ligulata.[Pubmed:
18622126]
Inhibitory compound of alpha-glucosidase activity, (+)-Afzelechin (1), was isolated from rhizomes of Bergenia ligulata.
METHODS AND RESULTS:
The structure was identified by IR, EI-MS, (1)H and (13)C NMR spectroscopy. The ID(50) (50% inhibition dose) value of compound 1 was 0.13 mM. To investigate the structure-activity relationship, (+)-Afzelechin tetraacetate (2), (+)-5,7,4'-trimethoxyafzelechin (3), (+)-tetramethoxyafzelechin (4), and (+)-3-acetyl-5,7,4'-trimethoxyafzelechin (5) as the derivatives of compound 1 were evaluated as well.