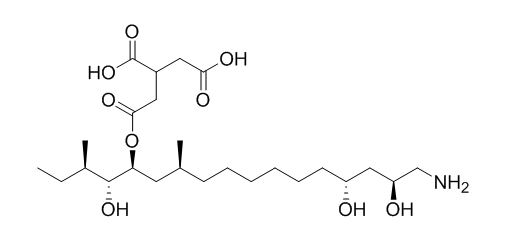
AAL Toxin TB1
AAL toxins TA and TB are phytotoxins, isolated from corn cultures by aqueous extraction. AAL-toxin is a potent natural herbicide, which disrupts sphingolipid metabolism of plants.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
EXCLI J.2023, 22:482-498.
ACS Pharmacol.Transl.Sci.2024, 4c00003.
Anesth Pain Med (Seoul).2020, 15(4):478-485.
Key Engineering Materials2022, 931(47-53).
Microchemical Journal2022, 182: 107874.
VNU J of Science: Med.&Pharm. Sci.2023, 39(1):20-29.
Molecules.2017, 22(11)
Antimicrob Agents Chemother.2020, AAC.01921-20.
J Ethnopharmacol.2023, 313:116534.
International. J. of Food Properties 2017, 20:S131-S140
Related and Featured Products
Pestic. Sci.,1995, 43, 181-187
AAL-toxin, a potent natural herbicide which disrupts sphingolipid metabolism of plants†[Reference:
WebLink]
METHODS AND RESULTS:
AAL-toxin, a natural product, has a wide range of phytotoxicity. It has potential as a natural herbicide because several important weeds including jimsonweed, black nightshade, prickly sida and hemp sesbania are quite sensitive, while some crops such as cotton and maize are not affected. This differential susceptibility may allow its exploitation for weed control.
CONCLUSIONS:
The data obtained to date a r e consistent with the hypothesis that the toxin disrupts sphingolipid metabolism and it is hoped that future studies will confirm these preliminary findings. More research is needed in the field to determine feasibility of commercial development of the toxin and its analogues as natural herbicides. Attention to human and mammalian toxicity will also be required.
Journal of Chromatography A,1993,641(1):95-100
Isolation and determination of AAL phytotoxins from corn cultures of the fungus Alternaria alternata f. sp. lycopersici[Reference:
WebLink]
METHODS AND RESULTS:
The fungus Alternaria alternata f. sp. lycopersici produces a group of four related host-specific phytotoxins (AAL toxins) which can be divided into two groups (TA and TB), each of which exists as an equilibrium mixture of two structural isomers. The AAL toxins were isolated from corn cultures by aqueous extraction, followed by purification on Amberlite XAD-2 resin, separation of TA from TB on silica gel and final purification on a semi-preparative high-performance liquid chromatographic (HPLC) system. A rapid, sensitive and reproducible method was developed to determine these toxins in culture material in order to monitor toxin production on corn cultures. The method consisted of aqueous extraction, C18 solid-phase extraction clean-up, precolumn derivatization with o-phthaldialdehyde and reversed-phase HPLC with fluorescence detection.
CONCLUSIONS:
An isocratic HPLC system was developed that separated the structural isomers of TA and TB within a chromatographic analysis time of 24 min.
Journal of Agricultural and Food Chemistry, 1994, 42(2):327-333.
Structural characterization of three new AAL toxins produced by Alternaria alternata f. sp. lycopersici.[Reference:
WebLink]
METHODS AND RESULTS:
Three new pairs of biologically active regioisomeric toxins (toxins TC, TD, and TE) were isolated from liquid cultures of alternata f.sp. lycopersici, purified using standard chromatographic procedures, and their structures elucidated following interpretation of spectra obtained from and mass spectrometry experiments. Each of the toxin congeners is structurally similar to the toxin TA, which was characterized earlier from culture filtrates of this fungus. toxin TC resembles TA but differs in its lack of at C4 and C5. toxin TE is the N-acetylated form of TC. Spectroscopic data are reported to confirm that TB is similar to TA but lacks a at C5, as suggested earlier. toxin TD is the N-acetylated form of TB.
CONCLUSIONS:
All five pairs of regioisomers arise as products of fungal and do not appear to be generated during isolation. All regioisomeric pairs induce the genotype-specific necrosis characteristic of toxin TA in bioassays but differ markedly in relative toxicity.