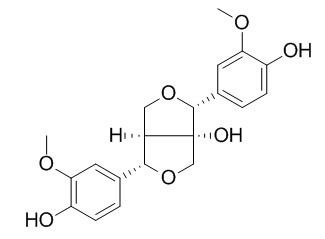
8-Hydroxypinoresinol
8-Hydroxypinoresinol has antioxidant activity,it and phillygenin significantly reduce the cell injury by 3-morpholinosydnonimine (SIN-1), a ONOO− generator, may be useful for the therapeutic or preventive applications in treating ONOO−-related diseases.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Med Food.2016, 19(12):1155-1165
J Med Food.2019, 22(10):1067-1077
J Anal Methods Chem.2022, 2022:2229500.
Molecules.2019, 24(9):E1719
Cells.2024, 13(14):1229.
Int J Mol Sci.2020, 21(24):9369.
Cells.2021, 10(11):2919.
Food Addit Contam Part A Chem Anal Control Expo Risk Assess.2020, 37(9):1437-1448.
Fermentation2023, 9(10), 889
Chem Biol Interact.2018, 290:44-51
Related and Featured Products
Phytother Res. 2009 Jul;23(7):938-42.
Cytoprotective effect of lignans from Forsythia suspensa against peroxynitrite-induced LLC-PK1 cell damage.[Pubmed:
19367664]
There is mounting evidence that peroxynitrite (ONOO(-)) is closely related to the pathogenesis of various diseases.
METHODS AND RESULTS:
As a pharmacological strategy aimed at preventing ONOO(-)-mediated toxicity, the protective activity of Forsythia suspensa (Thunb.) Vahl (Oleaceae) against ONOO(-)-induced cellular damage was investigated and its active components identified. After bioactivity-guided fractionation of its methylene chloride fraction, two tetrahydrofurofuran lignans were isolated, namely phillygenin and 8-Hydroxypinoresinol. The protective effects of these lignans against ONOO(-)-induced cell death were evaluated using renal epithelial cell LLC-PK1. Phillygenin and 8-Hydroxypinoresinol significantly reduced the cell injury by 3-morpholinosydnonimine (SIN-1), a ONOO(-) generator. The hydroxy substituents on the phenyl moieties may contribute to the antioxidant activities of these lignans.
CONCLUSIONS:
These results suggest that phillygenin and 8-Hydroxypinoresinol may be useful for the therapeutic or preventive applications in treating ONOO(-)-related diseases.
Arch Pharm Res. 2011 Dec;34(12):2029-35.
Lignans from the flowers of Osmanthus fragrans var. aurantiacus and their inhibition effect on NO production.[Pubmed:
22210027]
METHODS AND RESULTS:
A new lignan, (7R,7'R,8R,8'R)-8-Hydroxypinoresinol 8-O-β-D-glucopyranoside 4'-methyl ether (7), was isolated from the flowers of Osmanthus fragrans var. aurantiacus along with six known lignans: (+)-phillygenin (1), phillyrin (2), (-)-phillygenin (3), (-)-epipinoresinol-β-D-glucoside (4), taxiresinol (5), and (-)-olivil (6). The structure of the new compound was elucidated on the basis of 1D- and 2D-NMR spectroscopic analysis and specific rotation data. The compounds isolated from the flowers of O. fragrans var. aurantiacus were evaluated for inhibitory activities on nitric oxide production in lipopolysaccharide-stimulated macrophage RAW 264.7 cells. (+)-Phillygenin (1), phillyrin (2), and (-)-phillygenin (3) exerted the strongest inhibitory activities on NO production with IC(50) values of 25.5, 18.9, and 25.5 μM, respectively.
CONCLUSIONS:
These compounds may prove beneficial in the development of natural agents for prevention and treatment of inflammatory diseases.
Chem Biodivers. 2011 Oct;8(10):1908-13.
Sesquiterpenoids and lignans from the roots of Valeriana officinalis L.[Pubmed:
22006719]
METHODS AND RESULTS:
Two new guaiane-type sesquiterpenoids, valerol A (1) and kessyl 3-acetate (2), together with nine known compounds, valeracetate (3), anismol A (4), orientalol C (5), spatulenol (6), 4α,10α-epoxyaromadendrane (7), (+)-8-Hydroxypinoresinol (8), pinorespiol (9), pinoresinol 4-O-β-D-glucopyranoside (10), and 8-Hydroxypinoresinol 4'-O-β-D-glucopyranoside (11) were isolated from the roots of Valeriana officinalis.
CONCLUSIONS:
The structures and relative configurations of 1 and 2 were elucidated on the basis of spectroscopic methods (1D- and 2D-NMR, MS, UV, and IR). These compounds were evaluated for inhibitory activity on acetylcholinesterase (AChE) and enhancing activity on nerve growth factor (NGF)-mediated neurite outgrowth in PC12 cells.