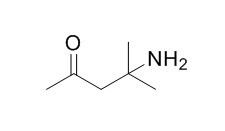
4-Amino-4-methyl-2-pentanone
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Int J Mol Sci.2024, 25(20):11227.
Braz J Med Biol Res. 2016, 49(7)
Key Engineering Materials2022, 931(47-53).
Journal of Oil Palm Research2019, 31(2):238-247
Natural Product Sciences2024, 30(4):254-261.
Reprod Toxicol.2020, 96:1-10.
Nat Prod Sci.2018, 24(2):109-114
Asian J of Pharmaceutical&Clinical 2018, 11(2)
Molecules.2023, 28(7):3039.
Horticulture Research2023, uhad164.
Related and Featured Products
Structural Chemistry, 2014, 25(3):707-714.
Transforming aspirin into novel molecular salts of salicylic acid.[Reference:
WebLink]
Aspirin is one of the most widely used analgesic, antipyretic, and anti-inflammatory drugs. Herein we disclose a way to transform aspirin into novel multicomponent crystal forms of salicylic acid, also a long-known analgesic with anti-inflammatory properties, among others, covering a broad spectrum of applications, including skin care products.
METHODS AND RESULTS:
A salicylic acid:salicylate ammonium salt and a salicylate:2-methyl-4-oxopentan-2-aminium molecular salt are concomitantly formed in acetone/ammonia solutions, resulting from aspirin decomposition. Furthermore the 2-methyl-4-oxopentan-2-aminium cation results from a sequence of in situ reactions: (i) imine formation, in which acetone is known to undergo under basic pH conditions; (ii) nucleophilic attack of α-carbon of the deprotonated acetone to the imine yielding 4-amino-4-methylpentan-2-one(4-Amino-4-methyl-2-pentanone); and (iii) protonation of 4-amino-4-methylpentan-2-one. In the structures obtained for the novel multicomponent crystal forms, the strong charge-assisted N+–H···O/O− hydrogen bonds between the drug molecule and the co-former play a key function in the supramolecular arrangement. The typical R22(8)carboxylic···carboxylic homosynthon observed in salicylic acid was inhibited by the salt formation.
CONCLUSIONS:
These results are in agreement with the results of a careful survey on the Cambridge Structural Database.