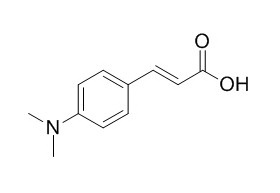
4-(Dimethylamino)cinnamic acid
4-(Dimethylamino)cinnamic acid (DMACA) could be as charge transfer (ICT) probe.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
JPC-Journal of Planar Chromatography 2017, 30(2)
J Mass Spectrom.2022, 57(2):e4810.
Front Pharmacol.2023, 14:1244655.
Front. Physiol.2022, 790345.
Plant Sci.2020, 301:110656.
APMIS.2019, 127(10):688-695
J Enzyme Inhib Med Chem.2019, 34(1):134-143
Natural Product Communications2021, 16(9):1-10.
Oncotarget.2015, 6(31):30831-49
Journal of Food Engineering2024, 379:112136.
Related and Featured Products
Spectrochim Acta A Mol Biomol Spectrosc. 2011 Mar;78(3):942-8.
Interaction of cinnamic acid derivatives with serum albumins: a fluorescence spectroscopic study.[Pubmed:
21247795]
Cinnamic acid (CA) derivatives are known to possess broad therapeutic applications including anti-tumor activity. The present study was designed to determine the underlying mechanism and thermodynamic parameters for the binding of two CA based intramolecular charge transfer (ICT) fluorescent probes, namely, 4-(dimethylamino) cinnamic acid (DMACA) and trans-ethyl p-(dimethylamino) cinnamate (EDAC), with albumins by fluorescence spectroscopy.
METHODS AND RESULTS:
Stern-Volmer analysis of the tryptophan fluorescence quenching data in presence of the added ligand reveals fluorescence quenching constant (κ(q)), Stern-Volmer constant (K(SV)) and also the ligand-protein association constant (K(a)). The thermodynamic parameters like enthalpy (ΔH) and entropy (ΔS) change corresponding to the ligand binding process were also estimated. The results show that the ligands bind into the sub-domain IIA of the proteins in 1:1 stoichiometry with an apparent binding constant value in the range of 10(4) dm(3) mol(-1).
CONCLUSIONS:
In both the cases, the spontaneous ligand binding to the proteins occur through entropy driven mechanism, although the interaction of DMACA is relatively stronger in comparison with EDAC. The temperature dependence of the binding constant indicates the induced change in protein secondary structure.
Photochem Photobiol Sci. 2008 Sep;7(9):1063-70.
Fluorimetric studies on the binding of 4-(dimethylamino)cinnamic acid with micelles and bovine serum albumin.[Pubmed:
18754053]
The constrained photophysics of intramolecular charge transfer (ICT) probe 4-(Dimethylamino)cinnamic acid (DMACA) was studied in different surfactant systems as well as in presence of model water soluble protein bovine serum albumin (BSA).
METHODS AND RESULTS:
Binding of the probe in ionic micelles like sodium dodecyl sulfate (SDS) and cetyl trimethyl ammonium bromide (CTAB) causes an increase in ICT fluorescence intensity, whereas, in non-ionic TritonX-100 (TX-100) the intensity decreases with a concomitant increase in emission from locally excited (LE) state. The observations were explained in terms of the different binding affinity, location of the probe and also the nature of specific hydrogen bonding interaction in the excited state nonradiative relaxation process of DMACA. The ICT fluorescence emission yield decreases in BSA due to the locking in of the probe buried in the hydrophobic pocket of the protein structure. SDS induced uncoiling of protein and massive cooperative binding between BSA and SDS is manifested by the release of probe molecules in relatively free aqueous environment.