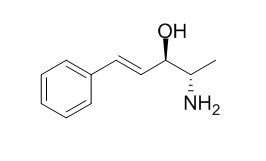
Merucathine
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Korean Soc Food Sci Nutr2023, 52(11):1101-1110
Journal of Research in Pharmacy.2022, 26(6):p1752-1757.
Hum. Ecol. Res.2025, 63(2):165-174
Bull. Pharm. Sci., Assiut University2020, 43(2):149-155.
J Ginseng Res.2023, 47(4):572-582.
J Agric Food Chem.2024, 72(42):23183-23195
J Sci Food Agric.2023, 103(1):213-220.
Br J Pharmacol.2018, 175(6):902-923
FEBS Lett.2021, 595(20):2608-2615.
ACS Omega.2024, 9(41):42227-42244.
Related and Featured Products
Anal Chem. 1994 Nov 15;66(22):4019-26.
Chiral resolution of cationic drugs of forensic interest by capillary electrophoresis with mixtures of neutral and anionic cyclodextrins.[Pubmed:
7810901]
METHODS AND RESULTS:
Chiral resolution of a number of cationic drugs of forensic interest (amphetamine, methamphetamine, cathinone, methcathinone, cathine, cocaine, propoxyphene, and various alpha-hydroxyphenethylamines) is achieved via capillary electrophoresis (CE) with added cyclodextrins (CDs), including novel mixtures of neutral and anionic CDs. In the latter studies, resolution and migration speed are readily adjusted by varying the ratio of the two added CDs, as the anionic CD acts as a counter-migrating complexing reagent. The neutral CD, heptakis(2,6-di-O-methyl)-beta-CD, was found suitable for the analysis of illicit cocaine and khat leaves (Catha edulis Forsk), which contain (-)-alpha-aminopropiophenone ((-)-cathinone), (+)-norpseudoephedrine (cathine), (-)-norephedrine, and trace levels of the phenylpentenylamines (+)-merucathinone, (+)-Merucathine, and possibly (-)-pseudoMerucathine.
CONCLUSIONS:
The use of mixtures of the neutral and the anionic CD (beta-CD sulfobutyl ether IV) was found suitable for the analysis of illicit amphetamine, methamphetamine, methcathinone, and propoxyphene. A model is presented for the impact of mixtures of neutral and anionic CDs on migration behavior and chiral resolution in CE.