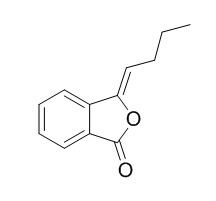
3-Butylidenephthalide
(Z)-3-butylidenephthalide has antihyperglycemic effect is due to inhibition of α-glucosidase at the intestinal level, inhibited the activity of yeast-α-glucosidase (IC(50) 2.35 mM) in a noncompetitive fashion with a K(i) of 4.86 mM. It can induce a dose-dependent antinociceptive action in the hot-plate assay, it is also effective for controlling the pain provoked by chemical irritation at the doses of 10 and 31.6 mg/kg.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Front Pharmacol.2021, 12:690113.
Food Chem X.2024, 24:101909.
Natural Product Sciences2023, 29(4):276-280.
J Pharm Pharmacol.2023, 75(9):1225-1236.
Journal of Research in Pharmacy.2022, 26(6):p1752-1757.
Nutrients.2020, 12(11):3448.
J Ethnopharmacol.2017, 206:327-336
Synthetic and Systems Biotechnology2023, j.synbio.
Int J Mol Sci.2018, 19(9):E2528
Elife.2021, 10:e68058.
Related and Featured Products
J Nat Prod. 2011 Mar 25;74(3):314-20.
(Z)-3-butylidenephthalide from Ligusticum porteri , an α-glucosidase inhibitor.[Pubmed:
20879744]
An extract from the roots of Ligusticum porteri, orally administered to groups of normal and diabetic mice, showed significant hypoglycemic and antihyperglycemic effects.
METHODS AND RESULTS:
Experimental type-II DM was achieved by treating mice with streptozotocin 15 min after an injection of β-nicotinamide adenine dinucleotide. (Z)-6,6',7,3'α-diligustilide (1), (Z)-ligustilide (2),(Z)-3-Butylidenephthalide , myristicin (4), and ferulic acid (5) were isolated from the active extract. When tested In Vivo, compounds 1-3 showed antihyperglycemic activity, with 3 being the most active. (Z)-3-Butylidenephthalide (56.2 mg/kg) decreased blood glucose levels in NAD-STZ-diabetic mice after an oral sucrose load, suggesting that its antihyperglycemic effect is due to inhibition of α-glucosidase at the intestinal level. Furthermore, (Z)-3-Butylidenephthalide inhibited the activity of yeast-α-glucosidase (IC(50) 2.35 mM) in a noncompetitive fashion with a K(i) of 4.86 mM. Docking analysis predicted that 3 binds to the enzyme in a pocket close to the catalytic site, but different from that for acarbose, with a K(i) of 11.48 mM. Compounds 1 and 2 did not affect α-glucosidase In Vivo, but altered glucose absorption by a mechanism yet to be determined.
CONCLUSIONS:
The stimulatory effect of 5 on insulin secretion, present in high amounts in the extract, has been demonstrated in previous investigations. The present study provides scientific support of the use of L. porteri in Mexican folk medicine for the treatment of diabetes.
Biopharm Drug Dispos. 2013 Sep;34(6):303-11.
Effects of natural phytochemicals in Angelica sinensis (Danggui) on Nrf2-mediated gene expression of phase II drug metabolizing enzymes and anti-inflammation.[Pubmed:
23640758]
The root of Angelica sinensis (Oliv.) Diels (abbreviated as AS) (Danggui) has a long history in Asian herbal medicine. Recently, it was demonstrated that AS possesses anti-cancer and anti-oxidant activities. Because the transcription factor Nrf2 mediates the expression of many cellular anti-oxidative stress genes, including genes that are involved in phase II drug metabolism and anti-oxidative stress, this study sought to investigate whether pure compounds from AS or an AS extract could activate antioxidant response element (ARE)-mediated gene expression and induce anti-inflammatory activities.
METHODS AND RESULTS:
Z-Ligustilide (Ligu), 3-Butylidenephthalide (Buty) and CO2 supercritical fluid-extracted lipophilic AS extract (SFE) were tested in HepG2-C8 cells stabilized with ARE luciferase reporter gene. Ligu and Buty caused significant toxicity only at 100 μm. All three samples induced ARE-luciferase activity; however, SFE at 8.5 μg/ml induced ARE-luciferase activity 2-3 fold more potently than did either of the pure compounds. SFE also significantly increased the endogenous mRNA of Nrf2 and the Nrf2 target anti-oxidative gene NAD(P)H dehydrogenase, quinone 1 (NQO1). The protein expression of NQO1 was also significantly induced by SFE. In RAW 264.7 cells, SFE suppressed lipopolysaccharide (LPS)-induced IL-1β and TNF-α expression about 2 fold stronger than sulforaphane, whereas both pure compounds and SFE suppressed inflammatory nitric oxide (NO) production.
CONCLUSIONS:
In summary, this study demonstrates that AS has anti-inflammatory effects and activates the Nrf2 pathway, which protects against oxidative stress.
Pharm Biol. 2014 Jan;52(1):14-20.
Antinociceptive activity of Ligusticum porteri preparations and compounds.[Pubmed:
24093628]
The roots and rhizomes of Ligusticum porteri Coulter & Rose (Apiaceae) are widely used in Mexican folk medicine for several purposes, including painful complaints. The main goal of this work was to demonstrate the analgesic action in mice of some preparations and major compounds from L. porteri.
METHODS AND RESULTS:
The extracts, aqueous (AE) and organic (OE), the essential oil (EO) and major compounds (10-316 mg/kg) from L. porteri were evaluated as potential antinociceptive agents using the acetic acid-induced writhing and hot plate tests in ICR mice. All preparations tested exhibited significant antinociceptive effect in the two animal pain models selected. AE and EO were more effective in the writhing test while OE had a better effect in the hot-plate model. On the other hand, Z-ligustilide (1) provoked an increment in the latency period to the thermal stimuli in the hot-plate test at a dose of 31.6 mg/kg, and a decrease in the number of abdominal writhes at 10 mg/kg. Z-3-Butylidenephthalide (2) induced a dose-dependent antinociceptive action in the hot-plate assay; this compound was also effective for controlling the pain provoked by chemical irritation at the doses of 10 and 31.6 mg/kg. Finally, diligustilide (3) inhibited the number of writhing responses at all doses tested but was inactive in the hot-plate model.
CONCLUSIONS:
The present investigation provides in vivo evidence supporting the use of L. porteri to treat painful conditions in folk medicine.
J Chromatogr A. 2013 Apr 5;1284:53-8.
Online isolation and purification of four phthalide compounds from Chuanxiong rhizoma using high-speed counter-current chromatography coupled with semi-preparative liquid chromatography.[Pubmed:
23484653]
The phthalide compounds of Chuanxiong rhizoma including senkyunolide A, levistolide A, Z-ligustilide and 3-Butylidenephthalide, have been reported as the biologically active compounds because of their therapeutic effects.
METHODS AND RESULTS:
In this work, online high-speed counter-current chromatography coupled with semi-preparative liquid chromatography instrument was set up, and online separation of the four compounds has been simultaneously achieved using this instrument. In this study, using all the selected solvent system, Z-ligustilide and 3-Butylidenephthalide were eluted in one peak by high-speed counter-current chromatography. Using high-speed counter-current chromatography with a solvent system of n-hexane-ethyl acetate-methanol-water-acetonitrile (8:2:5:5:5, v/v), 3.6 mg of senkyunolide A (94.4%) and 3.0mg of levistolide A (95.3%) were obtained from 100mg of the crude extract. Coeluted Z-ligustilide and 3-Butylidenephthalide peak fraction (8 mL) from high-speed counter-current chromatography was directly transferred and injected to the semi-preparative liquid chromatography for further separation. Finally, 5.6 mg of Z-ligustilide (97.5%) and 4.8 mg of 3-Butylidenephthalide (99.3%) were obtained.
CONCLUSIONS:
The identification of these four compounds was performed by quadrupole time-of-flight mass spectrometer, (1)H and (13)C nuclear magnetic resonance spectrometer.