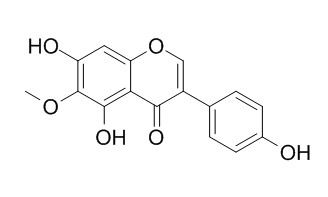
Tectorigenin
Tectorigenin has hepatoprotective, antifibrotic, anti-leukemia, antioxidant, and anti-inflammatory activities, it could sensitize paclitaxel-resistant human ovarian cancer cells through inactivation of the Akt/IKK/IκB/NFκB signaling pathway, and promise a new intervention to chemosensitize paclitaxel-induced cytotoxicity in ovarian cancer.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Pharmaceutics2022, 14(2),376.
Anticancer Res.2020, 40(10):5529-5538.
J of Applied Pharmaceutical Science2020, 10(1):077-082
Korean Journal of Pharmacognosy2018, 49(3):270-277
Plants (Basel).2021, 10(4):702.
Pharmacological Reports2020, 1-9
Plants (Basel).2023, 12(11):2107.
Preprints2021, doi:10.20944
J of Pharmaceutical Analysis2020, doi: 10.1016
Biomedicines.2024, 12(3):495.
Related and Featured Products
Pharm Biol. 2015 Apr 9:1-9.
Tectorigenin regulates adipogenic differentiation and adipocytokines secretion via PPARγ and IKK/NF-κB signaling.[Pubmed:
25856699]
Obesity is associated with a number of diseases with metabolic abnormalities such as type 2 diabetes (T2D). OBJECTIVE: We investigate the effects of Tectorigenin on 3T3-L1 preadipocyte differentiation and adipocytokines secretion.
METHODS AND RESULTS:
The effects of Tectorigenin on adipocyte differentiation were studied using Oil Red O staining. Effects of Tectorigenin on adipogenesis-related genes expression and adipocytokines secretion were measured by the real-time quantitative RT-PCR and ELISA method, respectively. Reporter gene assays were performed to determine the PPARγ and NF-κB transactivation. We also used [3H]-2-deoxy-d-glucose to study the glucose uptake, and the IKK/NF-κB signaling pathway was assessed by western blot analysis. HFD/STZ rats were used to evaluate the therapeutic efficacies of Tectorigenin. Tectorigenin 10, 25, 50, and 75 μM inhibited 3T3-L1 adipogenesis and related genes transcription. TNF-α-induced changes of IL-6, MCP-1, as well as adiponectin in 3T3-L1 were markedly reversed by Tectorigenin at 75 μM. Further investigation using reporter gene revealed that Tectorigenin was a partial PPARγ agonist with an IC50 value of 13.3 μM. Tectorigenin improved basal and insulin-stimulated glucose uptake in mature 3T3-L1 adipocytes. Moreover, Tectorigenin antagonized TNF-α-induced NF-κB transactivation and p65 nuclear translocation. Although Tectorigenin (50 and 100 mg/kg) displayed the ability to promote insulin sensitivity and improve glucose metabolism in HFD/STZ rats, it did not cause significant side effects such as body weight gain, fluid retention, or cardiac hypertrophy.
CONCLUSIONS:
These results suggest that Tectorigenin may ameliorate hyperglycemia by blocking preadipocyte differentiation and adipocytokines secretion in which PPARγ and NF-κB signaling pathways were involved.
Arch. Pharm. Res., 2008, 31(11):1447-56.
Tectorigenin inhibits IFN-γ/LPS-induced inflammatory responses in murine macrophage RAW 264.7 cells.[Reference:
WebLink]
Tectorigenin (Tg) and tectoridin (Td) are the major compounds isolated from the rhizomes of iridaceous plant Belamcanda chinensis which is well known as a chinese traditional medicine for the treatment of inflammatory diseases.
METHODS AND RESULTS:
In this study we investigated whether Tectorigenin and tectoridin can be applied to the suppression of interferon-γ and lipopolysaccharide (IFN-γ/LPS)-induced inflammatory responses in macrophages. Anti-inflammatory activities of Tectorigenin and tectoridin were compared with genistein (Ge), well known isoflavonoid as a phytoestrogen and regarded as an emerging anti-inflammatory agent. Both compounds showed low cytotoxic effect. In Raw 264.7 cells activated with IFN-γ/LPS, pre-treated Tectorigenin was found to inhibit the expression of inducible nitric oxide synthase (iNOS), the production of nitric oxide (NO) and the secretion of interleukin (IL)-1β dose-dependently. Tectorigenin also decreased the expression of cyclooxigenase (COX)-2 and the production of prostaglandin E2 (PGE2) in dose-dependent manner. These inhibitory effects of Tectorigenin were found to be caused by the blocking of nuclear factor kappa-B (NF-κB) activation. Compared with genistein and tectoridin, Tectorigenin showed significant inhibitory effect for almost anti-inflammatory tests in this study.
CONCLUSIONS:
All these results clearly demonstrated that Tectorigenin appears to have the potential to prevent inflammation.
Chin J Nat Med. 2014 Nov;12(11):841-6.
Tectorigenin inhibits the inflammation of LPS-induced acute lung injury in mice.[Pubmed:
25480515]
In a previous study, the anti-inflammatory effects of Tectorigenin were disclosed. In this study, the anti-inflammatory effects of Tectorigenin on acute lung injury using a lipopolysaccharide (LPS)-induced acute lung injury (ALI) mouse model were investigated.
METHODS AND RESULTS:
The cell-count in the bronchoalveolar lavage fluid (BALF) was measured. The animal lung edema degree was evaluated by the wet/dry weight (W/D) ratio. The superoxidase dismutase (SOD) activity and myeloperoxidase (MPO) activity was assayed using SOD and MPO kits, respectively. The levels of inflammatory mediators, including tumor necrosis factor-α (TNF-α), IL-1β, and IL-6 were assayed using an enzyme-linked immunosorbent assay method. Pathological changes of lung tissues were observed through HE staining. The inflammatory signal pathway related protein nuclear factor NF-κB p65 mRNA expression was measured by real-time PCR, and the protein level of NF-κB p65 was measured using Western blotting analysis. The data showed that treatment with the Tectorigenin markedly attenuated the inflammatory cell numbers in the BALF, decreased nuclear factor NF-κB p65 mRNA level and protein level in the lungs, and improved SOD activity and inhibited MPO activity. Histological studies showed that Tectorigenin substantially inhibited LPS-induced neutrophils in lung tissue compared with the model group.
CONCLUSIONS:
The results indicated that Tectorigenin had a protective effect on LPS-induced ALI in mice.
J Ethnopharmacol. 2010 Aug 19;131(1):165-73.
Tectorigenin inhibits the in vitro proliferation and enhances miR-338* expression of pulmonary fibroblasts in rats with idiopathic pulmonary fibrosis.[Pubmed:
20600766 ]
Tectorigenin is one of the main components in rhizomes of Iris tectorum, which is traditionally used to treat disorders such as hepatic cirrhosis caused by fibrosis. Idiopathic pulmonary fibrosis (IPF), one of the most common interstitial lung diseases, is caused by accumulation of fibroblasts in lungs.
In this work we sought to examine the effects of Tectorigenin on pulmonary fibroblasts in the IPF animal model and investigated the molecular mechanism (microRNA regulation) of Tectorigenin treatment.
METHODS AND RESULTS:
A well-known animal disease model of pulmonary fibrosis in rat was established by intratracheally instilling of bleomycin. In vitro cultured pulmonary fibroblasts in bleomycin-treated rats and in controls were treated with or without Tectorigenin. Comparative analyses of cell proliferation, apoptosis and cell cycle of pulmonary fibroblasts in bleomycin-treated rats and in controls were performed. Expression of miR-338* and its candidate gene LPA1 related to IPF of Tectorigenin-treated pulmonary fibroblasts in bleomycin-treated rats were further investigated.
Tectorigenin significantly inhibited the proliferation of pulmonary fibroblasts in bleomycin-treated rats but not in controls. However, no altered cell cycle and apoptosis of pulmonary fibroblasts in bleomycin-treated rats and in controls was observed after Tectorigenin treatment. Tectorigenin remarkably enhanced miR-338* expression of pulmonary fibroblasts in bleomycin-treated rats and downregulated LPA1 in the protein level.
CONCLUSIONS:
Tectorigenin inhibits the proliferation of pulmonary fibroblasts in vitro and enhances miR-338* expression, which might in turn downregulate LPA1. This indicates a potential inhibitory role of Tectorigenin on the pathogenesis of IPF.
Pharmacol Rep. 2015 Apr;67(2):382-7.
Tectorigenin ablates the inflammation-induced epithelial-mesenchymal transition in a co-culture model of human lung carcinoma.[Pubmed:
25712668]
Tumors not only manage to escape from the host immune system, but they effectively contrive to benefit from infiltrating immune cells by modifying their functions so as to create a pro-inflammatory microenvironment favorable for tumor progression and metastasis. In this study we investigated if Tectorigenin could suppress lung cancer-induced pro-inflammatory response generated from monocytes.
METHODS AND RESULTS:
A549:THP1 co-culture model was set-up favoring release of pro-inflammatory cytokines interleukin (IL)-6 and tumor necrosis factor alpha (TNF-α). Effect of Tectorigenin on A549 imparted invasive phenotype of A549:THP-1 co-culture was monitored by cytokine release from monocytes, and metastasis/epithelial-mesenchymal transitiom (EMT) in A549 cells. In a contact A549:THP1 co-culture model, THP-1 cells were activated by A549 cells favoring secretion of pro-inflammatory cytokines, TNF-α and IL-6. However, priming of A549 cells with Tectorigenin for 24h repressed A549 cell-induced secretion of TNF-α and IL-6 by THP-1 cells. Tectorigenin induced change in functional phenotype of A549 cells rendered THP-1 cells non-responsive for the secretion of IL-6 and TNF-α in a contact co-culture setup. Additionally, conditioned media from this non-responsive A549:THP-1 co-culture suppressed metastatic potential of A549 cells as confirmed by the wound healing and transwell migration assays. These finding were further corroborated by decrease in expression of Snail with a concomitant increase in E-cadherin, the two signature markers of EMT.
CONCLUSIONS:
These results clearly demonstrate the therapeutic potential of Tectorigenin to prevent lung cancer elicited inflammatory and pro-metastatic response in monocytes and warrants further investigations to elucidate its mechanism of action.
Carcinogenesis. 2012 Dec;33(12):2488-98.
Tectorigenin sensitizes paclitaxel-resistant human ovarian cancer cells through downregulation of the Akt and NFκB pathway.[Pubmed:
23027625 ]
Paclitaxel (Taxol) is currently used as the front-line chemotherapeutic agent for several cancers including ovarian carcinoma; however, the drug frequently induces drug resistance through multiple mechanisms. The new strategy of using natural compounds in combination therapies is highly attractive because those compounds may enhance the efficacy of chemotherapy.
METHODS AND RESULTS:
In this study, we found that Tectorigenin, an isoflavonoid isolated from flower of Pueraria thunbergiana, enhanced the growth-inhibitory effect of paclitaxel in paclitaxel-resistant ovarian cancer cells (MPSC1(TR), A2780(TR) and SKOV3(TR)) as well as their naive counterparts. The combination of Tectorigenin with paclitaxel resulted in a synergistic apoptosis compared with either agent alone through activation of caspases-3, -8 and -9. Treatment with Tectorigenin inhibited the nuclear translocation of NFκB and the expression of NFκB-dependent genes such as FLIP, XIAP, Bcl-2, Bcl-xL and COX-2, which are known to be associated with chemoresistance. In addition, the Tectorigenin-paclitaxel combination inhibited the phosphorylation of IκB and IKK and the activation of Akt in paclitaxel-resistant cancer cells. Moreover, Tectorigenin-paclitaxel-induced cell growth inhibition was enhanced by pretreatment with the Akt inhibitor LY294002 or overexpression of the dominant negative Akt (Akt-DN), but reduced by overexpression of constitutively activated Akt (Akt-Myr). Furthermore, we found that Akt-Myr, at least in part, reversed Tectorigenin-paclitaxel-induced nuclear translocation of NFκB and the phosphorylation of IκB and IKK.
CONCLUSIONS:
These data suggest that Tectorigenin could sensitize paclitaxel-resistant human ovarian cancer cells through inactivation of the Akt/IKK/IκB/NFκB signaling pathway, and promise a new intervention to chemosensitize paclitaxel-induced cytotoxicity in ovarian cancer.
J Pharmacol Sci. 2005 Apr;97(4):541-4.
Hepatoprotective effect of tectoridin and tectorigenin on tert-butyl hyperoxide-induced liver injury.[Pubmed:
15821336]
To clarify the hepatoprotective effects of tectoridin and Tectorigenin from Puerariae Flos, their effects on tert-butyl hyperoxide (t-BHP)-injured HepG2 cells and mice were investigated.
METHODS AND RESULTS:
When Tectorigenin at a dose of 50 mg/kg was intraperitoneally administered to mice injured by t-BHP, it significantly inhibited the increase the activities of plasma ALT and AST by 39% and 41%, respectively, in the t-BHP-treated group. The inhibitory effect of Tectorigenin is much more potent than that of a commercially available dimethyl diphenyl bicarboxylate. Orally administered tectoridin showed hepatoprotective activity. However, when tectoridin was intraperitoneally administrated to mice, no hepatoprotective activity was observed. Tectorigenin also protected against the cytotoxicity of HepG2 cells induced by t-BHP. This protection may have originated from the inhibition of apoptosis.
CONCLUSIONS:
Tectorigenin may be hepatoprotective and tectoridin should be a prodrug that is transformed to Tectorigenin.