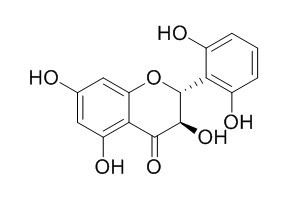
2',3,5,6',7-Pentahydroxyflavanone
(2R,3R)-2',3,5,6'7-pentahydroxyflavanone(2',3,5,6',7-Pentahydroxyflavanone) can inhibit the histamine release from rat mast cells induced by compound 48/80. It inhibits the lipid peroxide formation by Feand ascorbic acid, it also inhibits the lipid peroxide formation induced by adenosine diphosphate and reduces nicotinamide adenine dinucleotide phosphate in rat liver homogenate.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biomolecules.2024, 14(10):1257.
Front Pharmacol.2017, 8:205
Drug Dev Res.2020, doi: 10.1002
Neurochem Res.2021, s11064-021-03449-0
Phytother Res.2019, 33(7):1784-1793
Int Immunopharmacol.2020, 90:107268.
Cells. 2023, 12(15):1934.
Int J Nanomedicine.2024, 19:1683-1697.
Biomed Pharmacother.2024, 179:117410.
Plant Physiol Biochem.2019, 144:355-364
Related and Featured Products
Chem. Pharm. Bull., 1984, 32(12):5051-4.
Scutellariae radix. X. Inhibitory effects of various flavonoids on histamine release from rat peritoneal mast cells in vitro.[Reference:
WebLink]
The effects of various flavonoids isolated from the roots of Scutellaria baicalensis on the histamine release from mast cells were investigated.
METHODS AND RESULTS:
Many of the flavonoids (wogonin, wogonin-7-O-D-glucuronide, baicalein, skullcapflavanone II, (2S)-2',5,6',7-tetrahydroxyflavanone, (2R,3R)-2',3,5,6',7-Pentahydroxyflavanone and 2',5,5',7-tetrahydroxy-6'-8-dimethoxyflavone) inhibited the histamine release from rat mast cells induced by compound 48/80.
Chem Pharm Bull (Tokyo). 1982 May;30(5):1792-5.
Studies on Scutellariae radix. VI. Effects of flavanone compounds on lipid peroxidation in rat liver.[Reference:
WebLink]
Compounds (I and II) which inhibited lipid peroxides formation (in in vitro experiments) were isolated together with various flavonoids from the roots of Scutellaria baicalensis GEORGI.
METHODS AND RESULTS:
From the analytical and physical data, compounds I and II were identified as (2S)-2', 5, 6', 7-tetrahydroxyflavanone and (2R, 3R)-2',3,5,6',7-Pentahydroxyflavanone, respectively. Compound II inhibited the lipid peroxide formation by Feand ascorbic acid. Compounds I and II inhibited the lipid peroxide formation induced by adenosine diphosphate and reduced nicotinamide adenine dinucleotide phosphate in rat liver homogenate.
Yao Xue Xue Bao. 1989;24(3):200-6.
Studies on the structures of new flavonoids from the root of Scutellaria amoena.[Pubmed:
2816376]
From the root of Scutellaria amoena C.H. Wright, two new flavonoids (I, II) and six known flavonoids (III-VIII) were isolated.
METHODS AND RESULTS:
On the basis of spectroscopic analysis (UV, 1HNMR, 13CNMR, MS and CD) and chemical evidences, the structures of I and II were elucidated as (2S)-2',5,6'-trihydroxy-7-methoxyflavanone-2'-O-beta-D-glucopyrano side (I) and (2R, 3R)-2',3,5,7-tetrahydroxyflavanone (II) respectively. The other six known compounds were identified as (2S)-5,7,8-trihydroxyflavanone (III), (2S)-2',5,6',7-tetrahydroxyflavanone (IV), (2R, 3R)-2',3,5,6',7-Pentahydroxyflavanone (V), 2',5,6',7-tetrahydroxyflavone (VI) norwogonin (VII) and oroxylin-A (VIII) respectively.
CONCLUSIONS:
Compounds III-VIII were obtained from this plant for the first time.