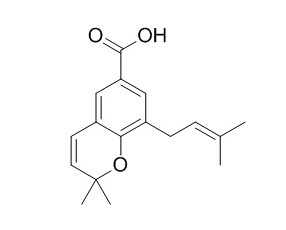
2,2-Dimethyl-8-prenylchromene 6-carboxylic acid
2,2-Dimethyl-8-prenylchromene 6-carboxylic acid showed antioxidative activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Oncotarget.2017, 8(53):90925-90947
J Biol Chem.2021, 297(6):101362.
Heliyon.2023, e12684.
J Appl Biol Chem.2021, 64(3),263?268
Vietnam Journal of Food Control2022, 5(3):pp.390-401.
Redox Biology2024, 103197.
J Agric Food Chem.2021, 69(11):3496-3510.
Neurochem Int.2020, 133:104629
Toxicol In Vitro.2022, 81:105346.
Evid Based Complement Alternat Med.2017, 2017:9764843
Related and Featured Products
Food Science and Technology Research, 2003, 9(2):197-201.
Antioxidative, Antihyaluronidase and Antityrosinase Activities of Some Constituents from the Aerial Part of Piper elongatum VAHL.[Reference:
WebLink]
METHODS AND RESULTS:
Seven known compounds, pyrroside B (1), swertisin (2), isovitexin (3), isoswertiajaponin (4), vomifoliol (blumenol A) (5), (6S,9R)-roseoside (6) and angelicoidenol (7) were isolated from the methanol (MeOH) extract of the aerial part of Piper elongatum VAHL. and their structures were identified on the basis of physical and spectral data. In addition, the antioxidative activity of 1–4 was evaluated by the ferric thiocyanate method.
CONCLUSIONS:
All these compounds showed stronger antioxidative activity than that of α-tocopherol. Furthermore, the scavenging effects on 1,1-diphenyl-2-picrylhydrazyl (DPPH), the antihyaluronidase and the antityrosinase activities of 1–4, asebogenin (8), 2′,6′-dihydroxy-4′-methoxydihydrochalcone (9), 3-geranyl-4-methoxybenzoic acid (10), 3-geranyl-4-hydroxybenzoic acid (11), nervogenic acid (12) and 2,2-dimethyl-6-carboxyl-8-prenyl-chromene (2,2-Dimethyl-8-prenylchromene 6-carboxylic acid,13), which were previously isolated from the MeOH extract were evaluated.
Compounds 4, 8 and 9 showed higher radical scavenging effect than that of L-cysteine, and 4, 8 and 11 exhibited stronger inhibition effect on the activation of hyaluronidase than that of tranilast. Compound 8 indicated almost the same antityrosinase activity as that of kojic acid.
Columbianetin beta-D-glucopyranoside
Catalog No: CFN95038
CAS No: 55836-35-6
Price: $288/5mg
Carasinol B
Catalog No: CFN95042
CAS No: 777857-86-0
Price: $333/5mg
3-O-[5'''-O-feruloyl-beta-D-apiofuranosyl(1'''->2'')-beta-D-glucopyranosyl] rhamnocitrin
Catalog No: CFN95154
CAS No: 148210-00-8
Price: $413/5mg
Geoside
Catalog No: CFN95233
CAS No: 585-90-0
Price: $368/5mg
Neoarctin B
Catalog No: CFN95243
CAS No: 155969-67-8
Price: $318/10mg
Vaccarin E
Catalog No: CFN95252
CAS No: 2252345-81-4
Price: $318/10mg
(3beta,22alpha)-26-(beta-glucopyranosyloxy)-22-methoxyfurost-5-en-3-yl 2-O-(6-deoxy-alpha-mannopyranosyl)-beta-glucopyranosiduronic acid
Catalog No: CFN95321
CAS No: 107783-53-9
Price: $318/20mg
1,3,4,6-Tetragalloylglucose
Catalog No: CFN95425
CAS No: 26922-99-6
Price: $318/10mg
12beta-Acetoxy-3,7,11,15,23-pentaoxo-lanost-8,20-dien-26-oic acid
Catalog No: CFN95505
CAS No: 1309931-91-6
Price: $318/5mg
8-Hydroxy-5,7-dimethoxyflavanone
Catalog No: CFN95580
CAS No: 201230-40-2
Price: $318/5mg