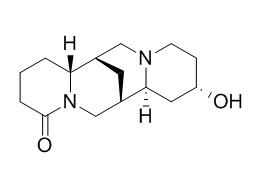
13-Hydroxylupanine
13-Hydroxylupanine is a natural product from Cytisus scoparius.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
J Cell Mol Med.2022, 26(23):5807-5819.
Comput Biol Chem.2019, 83:107096
Wageningen University & Research2018, January 2018
Front Microbiol.2022, 12:833233.
Front Plant Sci.2021, 12:673337.
Inflammation2015, 38(1):445-55
Int J Mol Sci.2018, 19(9):E2681
VNU J Science: Med.&Pharm. Sci.2024.2588-1132
University of Limpopo2016, 1777
Cells.2023, 12(1):168.
Related and Featured Products
Planta Med. 1983 Aug;48(8):253-7.
Accumulation of quinolizidine alkaloids in plants and cell suspension cultures: genera lupinus, cytisus, baptisia, genista, laburnum, and sophora.[Pubmed:
17404991]
The patterns of quinolizidine alkaloids in cell cultures of 10 species of Fabaceae were analyzed by high-resolution GLC and GLC-MS and compared with the alkaloids present in the leaves of the respective plants.
METHODS AND RESULTS:
Lupanine was produced in all 10 cell suspension cultures as the main alkaloid. It was accompanied by sparteine, tetrahydrorhombifoline, 17-oxosparteine, 13-Hydroxylupanine, 4-hydroxylupanine, 17-oxolupanine, and 13-Hydroxylupanine esters as minor alkaloids in some species. The alkaloid patterns of the plants differed markedly in that alpha-pyridone alkaloids were the major alkaloids in the genera Cytisus, Genista, Laburnum and Sophora but were not accumulated in the cell cultures.
CONCLUSIONS:
These data further support the assumption that the pathway leading to lupanine is the basic pathway of quinolizidine alkaloids biosynthesis and that the other alkaloids are derived from lupanine.
Xenobiotica. 1994 Sep;24(9):933-41.
Disposition of lupanine and 13-hydroxylupanine in man.[Pubmed:
7810174]
1. The in vivo disposition of lupanine and 13-Hydroxylupanine was studied in subjects identified as poor metabolizers (PM, n = 4) and extensive metabolizers (EM, n = 7) phenotypes for cytochrome P4502D6 (CYP2D6).
METHODS AND RESULTS:
2. After oral administration (40.26 mumol), the half-life (t1/2) of lupanine determined from urinary excretion rate studies in EM subjects was 6.2 +/- 0.5 h (mean +/- SEM) with 95.5 +/- 6.0% of the dose recovered unchanged within 72 h. Similarly, in PM subjects t1/2 = 6.5 +/- 0.9 h and recovery 89.9 +/- 4.5%. 3. For orally administered 13-Hydroxylupanine (37.83 mumol) the t1/2 in EM subjects was 6.8 +/- 1.0 h with a recovery of 100.5 +/- 5.3%, and in PM subjects t1/2 = 5.9 +/- 1.6 h with a recovery of 102.5 +/- 4.8%. 4. The t1/2s of both lupanine and 13-Hydroxylupanine respectively did not differ significantly between EM and PM phenotypes. In addition, total recovery of dose for both alkaloids was similar between phenotypes. 5. In most subjects, > 76% of lupanine and > 85% of 13-Hydroxylupanine was recovered as the unchanged compound. Significant apparent partial dehydroxylation of 13-hydroxy-lupanine was observed in one EM (14% of dose) and one PM (34% of dose) subject.
CONCLUSIONS:
6. Overall, the finding of a high urinary recovery of unchanged lupanine or 13-Hydroxylupanine together with similar t1/2s for both alkaloids in EM and PM CYP2D6 phenotypes suggests that clinical toxicity is unlikely to result from the use of lupin seed in footstuffs.