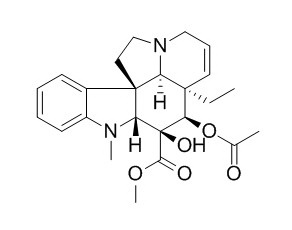
Vindorosine
Vindorosine has blood vessel relaxation effect, possible underlying mechanisms involving the inhibition of Ca(2+) entry via L-type Ca(2+) channels in vascular smooth muscles.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Acta horticulturae2017, 1158:257-268
Molecules.2022, 27(5):1675
J Sep Sci.2021, 44(22):4064-4081.
Drug Dev Res.2020, doi: 10.1002
Molecules.2023, 28(13):4907.
JAOCS2021, 98(7):779-794.
Natural Product Communications2022, 7(3):1-7.
Int J Mol Sci.2015, 16(8):18396-411
Research Square2021, 10.21203.
Food Chemistry: X2023, 101032.
Related and Featured Products
Planta Med. 2014 Dec;80(18):1672-7.
Antagonism of Ca2+ influx via L-type Ca2+ channels mediates the vasorelaxant effect of Catharanthus roseus-derived vindorosine in rat renal artery.[Pubmed:
25340466]
The present study examined possible cellular mechanisms for the relaxation of rat renal arteries induced by Vindorosine extracted from C. roseus.
METHODS AND RESULTS:
Intrarenal arteries were isolated from 200-300 g male Sprague-Dawley rats and treated with different pharmacological blockers and inhibitors for the measurement of vascular reactivity on a Multi Myograph System. Fluorescence imaging by laser scanning confocal microscopy was utilized to determine the intracellular Ca(2+) level in the vascular smooth muscles of the renal arteries. Vindorosine in micromolar concentrations relaxes renal arteries precontracted by KCl, phenylephrine, 11-dideoxy-9α,11α-epoxymethanoprostaglandin F2α, and serotonin. Vindorosine-induced relaxations were unaffected by endothelium denudation or by treatment with the nitric oxide synthase inhibitor N (G)-nitro-L-arginine methyl ester hydrochloride, the guanylyl cyclase inhibitor 1H-[1, 2, 4]oxadiazolo[4,3-a]quinoxalin-1-one, the cyclooxygenase inhibitor indomethacin, or K(+) channel blockers such as tetraethylammonium ions, glibenclamide, and BaCl2. Vindorosine-induced relaxations were attenuated in the presence of 0.1 µM nifedipine (an L-type Ca(2+) channel blocker). Vindorosine also concentration-dependently suppressed contractions induced by CaCl2 (0.01-5 mM) in Ca-free 60 mM KCl solution. Furthermore, fluorescence imaging using fluo-4 demonstrated that 30 min incubation with 100 µM Vindorosine reduced the 60 mM KCl-stimulated Ca(2+) influx in the smooth muscles of rat renal arteries.
CONCLUSIONS:
The present study is probably the first report of blood vessel relaxation by Vindorosine and the possible underlying mechanisms involving the inhibition of Ca(2+) entry via L-type Ca(2+) channels in vascular smooth muscles.
J Am Chem Soc. 2010 Sep 29;132(38):13533-44.
Asymmetric total synthesis of vindorosine, vindoline, and key vinblastine analogues.[Pubmed:
20809620]
METHODS AND RESULTS:
Concise asymmetric total syntheses of vindoline (1) and Vindorosine (2) are detailed based on a unique intramolecular [4 + 2]/[3 + 2] cycloaddition cascade of 1,3,4-oxadiazoles inspired by the natural product structures.Implementation of the approach for the synthesis of 1 and Vindorosine required the development of a ring expansion reaction to provide a 6-membered ring suitably functionalized for introduction of the Δ(6,7)-double bond found in the core structure of the natural products.
CONCLUSIONS:
Two unique approaches were developed that defined our use of a protected hydroxymethyl group as the substituent that controls the stereochemical course of the cycloaddition cascade.