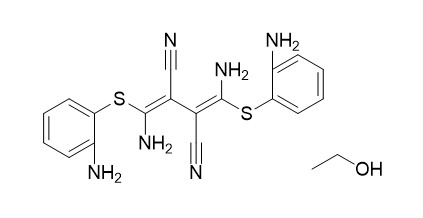
U0126-EtOH
U0126-EtOH is a highly selective inhibitor of MEK1/2 with IC50 of 0.07 μM/0.06 μM in cell-free assays, 100-fold higher affinity for ΔN3-S218E/S222D MEK than PD98059. U0126 inhibits autophagy and mitophagy with antiviral activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Pharmacol Rep.2022, 74(1):175-188.
Inflammation2015, 38(1):445-55
Int. J. Mol. Sci. 2022, 23(3),1696.
Revista Brasileira de Farmacognosia2024, 34:1091-1100.
Nutrients.2024, 16(16):2612.
Regul Toxicol Pharmacol.2023, 142:105433.
Int J Mol Med.2016, 37(2):501-8
Molecules.2019, 24(17):E3127
J Chromatogr B Analyt Technol Biomed Life Sci. 2017, 1064:115-123
Anal Biochem.2019, 569:10-15
Related and Featured Products
Cancer Biol Ther,2011 Dec 1;12(11):966-77.
A metabolic perturbation by U0126 identifies a role for glutamine in resveratrol-induced cell death.[Pubmed:
22108021]
Recent evidence has identified substantial overlap between metabolic and oncogenic biochemical pathways, suggesting novel approaches to cancer intervention. For example, cholesterol lowering statins and the antidiabetes medication metformin both act as chemopreventive agents in prostate and other cancers. The natural compound resveratrol has similar properties: increasing insulin sensitivity, suppressing adipogenesis, and inducing apoptotic death of cancer cells in vitro. However, in vivo tumor xenografts acquire resistance to resveratrol by an unknown mechanism, while mouse models of metabolic disorders respond more consistently to the compound.
METHODS AND RESULTS:
Here we demonstrate that castration-resistant human prostate cancer C4-2 cells are more sensitive to resveratrol-induced apoptosis than isogenic androgen-dependent LNCaP cells. The MEK inhibitor U0126 antagonized resveratrol-induced apoptosis in C4-2 cells, but this effect was not seen with other MEK inhibitors. U0126 was found to inhibit mitochondrial function and shift cells to aerobic glycolysis independently of MEK. Mitochondrial activity of U0126 arose through decomposition, producing both mitochondrial fluorescence and cyanide, a known inhibitor of complex IV. Applying U0126 mitochondrial inhibition to C4-2 cell apoptosis, we tested the possibility that glutamine supplementation of citric acid cycle intermediate α-ketoglutarate may be involved.
CONCLUSIONS:
Suppression of the conversion of glutamate to α-ketoglutarate antagonized resveratrol-induced death in C4-2 cells.
A similar effect was also seen by reducing extracellular glutamine concentration in the culture medium, suggesting that resveratrol-induced death is dependent on glutamine metabolism, a process frequently dysregulated in cancer. Further work on resveratrol and metabolism in cancer is warranted to ascertain if the glutamine dependence has clinical implications.
Behav Brain Res,2012 Jun 15;232(1):165-73.
ERK and p38 inhibitors attenuate memory deficits and increase CREB phosphorylation and PGC-1α levels in Aβ-injected rats.[Pubmed:
22510382]
METHODS AND RESULTS:
In this study, we investigated the effect of intracerebroventricular administration of ERK and p38 specific inhibitors, U0126 and PD169316, respectively, on learning and memory deficits induced by amyloid beta (Aβ) in rats. To investigate the effects of these compounds on learning and memory, we performed Morris water maze (MWM) test. U0126 and/or PD169316 improved spatial learning in MWM in Aβ-injected rats, 20 days after Aβ-injection. To determine the mechanisms of action of U0126 and PD169316, we studies their effect on some intracellular signaling pathways such as Ca(+)/cAMP-response element binding protein (CREB), c-fos, and transcription factors that regulate mitochondrial biogenesis. Based on our data, CREB and c-fos levels decreased 7 days after Aβ-injection, while U0126 and/or PD169316 pretreatments significantly increased these levels. Moreover, U0126 and PD169316 activated peroxisome proliferator-activated receptor gamma coactivator-1a, nuclear respiratory factor 1, and mitochondrial transcription factor A, 7 days after Aβ-injection. Surprisingly, these factors were returned to vehicle level, 20 days after Aβ-injection.
CONCLUSIONS:
Our findings reinforce the potential neuroprotective effect of these inhibitors against the Aβ toxicity.
Bioorg Med Chem Lett,1998 Oct 20;8(20):2839-44.
MEK inhibitors: the chemistry and biological activity of U0126, its analogs, and cyclization products.[Pubmed:
9873633]
METHODS AND RESULTS:
In search of antiinflammatory drugs with a new mechanism of action, U0126 was found to functionally antagonize AP-1 transcriptional activity via noncompetitive inhibition of the dual specificity kinase MEK with an IC50 of 0.07 microM for MEK 1 and 0.06 microM for MEK 2.
CONCLUSIONS:
U0126 can undergo isomerization and cyclization reactions to form a variety of products, both chemically and in vivo, all of which exhibit less affinity for MEK and lower inhibition of AP-1 activity than parent, U0126.
J Immunol,1998 May 1;160(9):4175-81.
Inhibition of mitogen-activated protein kinase kinase blocks T cell proliferation but does not induce or prevent anergy.[Pubmed:
9574517]
Cell lines:A.E7 or Th17 cells
Concentrations: 0 to 10 μM
Incubation Time: 48 hours-
Method:
A.E7 or Th17 cells are incubated with mitomycin C-treated B10.BR or BALB/c splenocytes plus varying concentrations of pigeon cytochrome c or PR8 Ag, or with 5 U/mL human rIL-2. In addition, some assays contains U0126 or an inactive analogue, U0124, to determine direct effects of MEK inhibition on T cell proliferation. Two days after culture initiation, each well is pulsed with 1 µCi of [3H]TdR and harvested the following day. The incorporation of [3H]TdR into DNA is quantitated on a Packard Matrix 96 direct beta counter without the use of liquid scintillation mixtures.
Antiviral Res,2011 Nov;92(2):195-203.
Antiviral activity of the MEK-inhibitor U0126 against pandemic H1N1v and highly pathogenic avian influenza virus in vitro and in vivo.[Pubmed:
21854809]
Animal Models: Female C57Bl/6 mice infected by Mouse-adapted highly pathogenic avian influenza A/FPV/Bratislava/79 (H7N7; FPV) virus and swine origin human influenza A virus (SOIV) A/Regensburg/D6/2009 (H1N1v; RB1).
Formulation: ≤10 mM
Administration: Administered via aerosol.