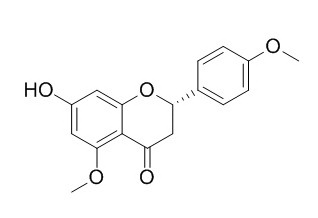
Tsugafolin
Tsugafolin has weak anti-HIV activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Nutrients.2018, 10(12):E1998
Current Analytical Chemistry2024, 20(8):599-610.
Korean Herb. Med. Inf.2021, 9(2):231-239.
Biomolecules.2020, 10(6):925.
Biomolecules2021, 11(10),1513.
Int J Mol Sci.2017, 19(1)
J. Food Composition and Anal.2022, V 109:104482.
Hum Exp Toxicol.2022, 41:9603271221143713.
Hortic Res.2023, 10(9):uhad154.
JMicrobiol Biotech Food Sci2021, e4289.
Related and Featured Products
J Nat Prod. 2014 Mar 28;77(3):663-7.
Bioactive compounds from Vitex leptobotrys.[Pubmed:
24404757]
METHODS AND RESULTS:
A new lignan, vitexkarinol (1), as well as a known lignan, neopaulownin (2), a known chalcone, 3-(4-hydroxyphenyl)-1-(2,4,6-trimethoxyphenyl)-2-propen-1-one (3), two known dehydroflavones, Tsugafolin (4) and alpinetin (5), two known dipeptides, aurantiamide and aurantiamide acetate, a known sesquiterpene, vemopolyanthofuran, and five known carotenoid metabolites, vomifoliol, dihydrovomifoliol, dehydrovomifoliol, loliolide, and isololiolide, were isolated from the leaves and twigs of Vitex leptobotrys through bioassay-guided fractionation. The chalcone (3) was found to inhibit HIV-1 replication by 77% at 15.9 μM, and the two dehydroflavones (4 and 5) showed weak anti-HIV activity with IC50 values of 118 and 130 μM, respectively, while being devoid of cytotoxicity at 150 μM.
CONCLUSIONS:
A chlorophyll-enriched fraction of V. leptobotrys, containing pheophorbide a, was found to inhibit the replication of HIV-1 by 80% at a concentration of 10 μg/mL. Compounds 1 and 3 were further selected to be evaluated against 21 viral targets available at NIAID (National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD, USA).
Phytochemistry. 2000 Nov;55(5):439-46.
(Rel)-1beta,2alpha-di-(2,4-dihydroxy-6-methoxybenzoyl)-3beta, 4alpha-di-(4-methoxyphenyl)-cyclobutane and other flavonoids from the aerial parts of Goniothalamus gardneri and Goniothalamus thwaitesii.[Pubmed:
11140605]
METHODS AND RESULTS:
The aerial parts of Goniothalamus gardneri (Annonaceae) has yielded the known flavonoids 2'-hydroxy-4,4',6'-trimethoxychalcone (flavokawain A), 2',4'-dihydroxy-4,6'-dimethoxydihydrochalcone, 4,2',4'-trihydroxy-6'-methoxydihydrochalcone, 5,7,4'-trimethoxyflavanone (naringenin trimethyl ether) and 7-hydroxy-5,4'-dimethoxyflavanone (Tsugafolin) together with three novel compounds, the dimer characterised as (rel)-1beta,2alpha-di-(2,4-dihydroxy-6-methoxybenzoyl)-3beta,4alpha-di-(4-methoxyphenyl)-cyclobutane, 2',4'-dihydroxy-4,6'-dimethoxychalcone and 2'-hydroxy-4,4',6'-trimethoxydihydrochalcone. The last two have previously been synthesised but appear to be new natural products.
CONCLUSIONS:
A similar study of the aerial parts of G. thwaitesii led only to the isolation of the known flavonoids myricetin 4'-O-methyl ether-3-O-alpha-L-rhamnopyranoside (mearnsitrin) and myricetin-3-O-methyl ether (annulatin), together with the triterpenes friedelinol, friedelin and betulinic acid. All compounds were identified by spectroscopic analysis and, for known compounds, by comparison with published data.