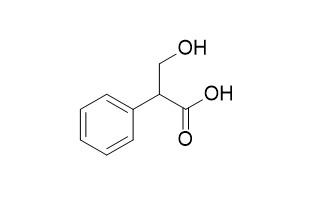
Tropic acid
Reference standards.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Phytomedicine.2017, 24:77-86
Journal of Functional Foods2022, 98:105271.
Front Microbiol.2021, 12:736780.
Nutrients.2020, 12(12):3607.
Agronomy2023, 13(6), 1435.
VNU J Science: Med.&Pharm. Sci.2023, 39(2):43-52.
Nat Commun.2025, 16(1):4121.
Genes (Basel).2021, 12(7):1024.
Appl. Sci.2023, 13(2), 860.
Psychopharmacology (Berl).2020, 10.1007
Related and Featured Products
Naunyn-Schmiedeberg's archives of pharmacology, 2006, 373(3):230.
The presence of atropinesterase activity in animal plasma.[Reference:
WebLink]
The enzyme atropinesterase (EC 3.1.1.10) causes the rapid hydrolysis of tropane alkaloids such as atropine and scopolamine.
METHODS AND RESULTS:
This enzyme is known to occur in a certain proportion of rabbits and some plants, although its presence in other animal species remains controversial. The potential presence in some animals but not others of an enzyme which can rapidly hydrolyse compounds such as atropine is a potential unwanted experimental variable in many experiments. Because of the uncertainty surrounding the enzyme and the paucity of data, it was decided to examine whether we could detect and characterise atropinesterase activity in the plasma of dogs, goats, guinea-pigs, humans, pigs, rabbits and rhesus by separating and quantitating the substrate (atropine) and one of the products (Tropic acid) by high performance liquid chromatography (HPLC).
CONCLUSIONS:
It was found that plasma from some but not all rabbits possessed a capacity to breakdown large quantities of atropine; an effect that was apparently enantiomer-specific. Plasma from other rabbits, and plasma from all other species investigated, proved capable of hydrolysing atropine at a rate exceeding that of non-specific breakdown. It remains to be determined whether this effect is due to a low expression of atropinesterase or an alternative hydrolysing enzyme.
Journal of Separation ence, 2015, 32(17):2864-2870.
Occurrence and behavior of system peaks in RP HPLC with solely aqueous mobile phases.[Reference:
WebLink]
System peaks are important but often also disturbing phenomena occurring in separation systems.
METHODS AND RESULTS:
Behavior of system peaks was studied in reversed phase high performance liquid chromatography (RP HPLC) systems consisting of an RP Amide C16 column and aqueous solutions of organic acids with alkaline metal hydroxides as mobile phases. Binary mobile phases, composed of benzoic acid and lithium hydroxide (LiOH) or cesium hydroxide (CsOH), yielded two system peaks. The first peak was stationary and the second one moved with dilution of the mobile phase or with changes of the alkaline metal hydroxide concentration. The latter changes affected dissociation of the benzoic acid present in the mobile phase and thereby its retention. The presumption that the first system peak is not influenced by the type of alkaline metal cation and that it is related to the non-adsorbed component of the mobile phase was confirmed by a cyclic procedure.
CONCLUSIONS:
Three-component mobile phases composed of benzoic acid, Tropic acid, and a hydroxide gave rise to three system peaks as expected. The first peak was again stationary and the two others shifted depending on the concentration variation of both acids. Resonance causing a zigzag peak, well described in capillary zone electrophoresis (CZE), was observed if 1-pentanol was injected into a chromatographic system with one-component mobile phase.