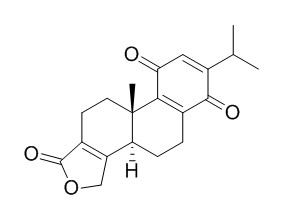
Triptoquinonide
Triptoquinonide is a natural product from Tripterygium wilfordii.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Appl. Sci.2020, 10(4),1304
APMIS.2019, 127(10):688-695
Nutr Cancer.2022, 1-13.
Molecules.2019, 24(21):E3834
Molecules2022, 27(14):4601
J Korean Soc Food Sci Nutr2023, 52(11):1101-1110
Molecules.2024, 29(3):671.
J Appl Biol Chem2022, 65:343−348.
J Sci Food Agric.2023, 103(1):213-220.
Biomolecules2021, 11(10),1513.
Related and Featured Products
J Org Chem. 2000 Apr 7;65(7):2208-17.
Enantioselective total synthesis of (-)-triptolide, (-)-triptonide, (+)-triptophenolide, and (+)-triptoquinonide.[Pubmed:
10774048]
The first enantioselective total synthesis of (-)-triptolide (1), (-)-triptonide (2), (+)-triptophenolide (3), and (+)-Triptoquinonide (4) was completed.
METHODS AND RESULTS:
The key step involves lanthanide triflate-catalyzed oxidative radical cyclization of (+)-8-phenylmenthyl ester 30 mediated by Mn(OAc)3, providing intermediate 31 with good chemical yield (77%) and excellent diastereoselectivity (dr 38:1). (+)-Triptophenolide methyl ether (5) was then prepared in > 99% enantiomeric excess (> 99% ee), and readily converted to natural products 1-4. In addition, transition state models were proposed to explain the opposite chiral induction observed in the oxidative radical cyclization reactions of chiral beta-keto esters 17 (without an alpha-substituent) and 17a (with an alpha-chloro substituent).