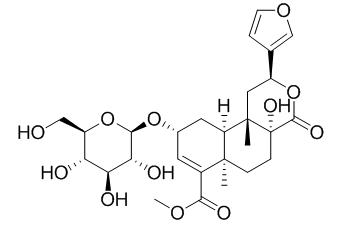
Tinospinoside C shows inhibitory activities of NO production with the IC(50) value of 218 uM.
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Planta Med. 2012 Jan;78(1):82-5
Clerodane diterpenoids from Tinospora sagittata (Oliv.) Gagnep.[Pubmed:
21969117 ]
METHODS AND RESULTS:
Three new clerodane diterpene glycosides, tinospinosides A (1), B (2), and C (3) were isolated from the roots of Tinospora sagittata (Oliv.) Gagnep. Their structures were determined to be (2 S,4a R,6a R,9 R,10a S,10b S)-2-(3-furanyl)-9-( β-D-glucopyranosyloxy)-1,4,4a,5,6,6a,9,10,10a,10b-decahydro-6a,10b-dimethyl-4-oxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (1), (2 S,4a S,6a R,9 R,10a R,10b S)-2-(3-furanyl)-9-( β-D-glucopyranosyloxy)-1,4,4a,5,6,6a,9,10,10a,10b-decahydro-4a-hydroxyl-6a,10b-dimethyl-4-oxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (2) and (2 S,4a R,6a R,9 R,10a R,10b S)-2-(3-furanyl)-9-( β-D-glucopyranosyloxy)-1,4,4a,5,6,6a,9,10,10a,10b-decahydro-4a-hydroxyl-6a,10b-dimethyl-4-oxo-2H-naphtho[2,1-c]pyran-7-carboxylic acid methyl ester (3), by various spectroscopic analyses, chemical reactions, and computer-assisted calculations.
The inhibitory activities of NO production by these compounds and their chemical derivatives in lipopolysaccharide and TNF γ-activated macrophage-like cell line J774.1 were tested.
CONCLUSIONS:
Tinospin A, 12- EPI-tinospin A, tinospinoside B, and Tinospinoside C showed inhibitory activities of NO production with the IC(50) values of 162, 182, 290, and 218 μM, respectively.
Planta Med. 2014 Mar;80(5):419-25.
New clerodane diterpenes from Tinospora sagittata var. yunnanensis.[Pubmed:
24634023]
METHODS AND RESULTS:
Four new clerodane diterpenes, namely sagittatayunnanosides A-D (1-4), were isolated from the roots of Tinospora sagittata var. yunnanensis, together with two known compounds, Tinospinoside C (5) and tinospinoside E (6).
CONCLUSIONS:
The structures of the four new compounds were well elucidated by extensive analyses of the MS, IR, and 1D and 2D NMR data. The cytotoxic and antifouling activities of compounds 1-6 were evaluated.