Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
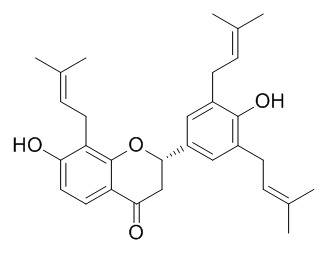
Journal of Separation Science, 2014, 37(22):3235-44.
Simultaneous determination of trifolirhizin, (-)-maackiain, (-)-sophoranone, and 2-(2,4-dihydroxyphenyl)-5,6-methylenedioxybenzofuran from Sophora tonkinensis in rat plasma by liquid chromatography with tandem mass spectrometry and its application to a ph[Reference:
WebLink]
METHODS AND RESULTS:
A new liquid chromatography with tandem mass spectrometry method was developed and validated for the simultaneous determination of trifolirhizin, (-)-maackiain, (-)-Sophoranone, and 2-(2,4-dihydroxyphenyl)-5,6-methylenedioxybenzofuran from Sophora tonkinensis in rat plasma using chlorpropamide as an internal standard. Plasma samples (50 uL) were prepared using a simple deproteinization procedure with 150 uL of acetonitrile containing 100 ng/mL of chlorpropamide. Chromatographic separation was carried out on an Acclaim RSLC120 C18 column (2.1 * 100 mm, 2.2 um) using a gradient elution consisting of 7.5 mM ammonium acetate and acetonitrile containing 0.1% formic acid (0.4 mL/min flow rate, 7.0 min total run time). The detection and quantitation of all analytes were performed in selected reaction monitoring mode under both positive and negative electrospray ionization. This assay was linear over concentration ranges of 50-5000 ng/mL (trifolirhizin), 25-2500ng/mL ((-)-maackiain), 5-250ng/mL ((-)-Sophoranone), and 1-250ng/mL 2-(2,4-dihydroxyphenyl)-5,6-methylenedioxybenzofuran) with a lower limit of quantification of 50, 25, 5, and 1 ng/mL for trifolirhizin, (-)-maackiain, (-)-Sophoranone, and 2-(2,4-dihydroxyphenyl)-5,6-methylenedioxybenzofuran, respectively.
All the validation data, including the specificity, precision, accuracy, recovery, and stability conformed to the acceptance requirements.
CONCLUSIONS:
The results indicated that the developed method is sufficiently reliable for the pharmacokinetic study of the analytes following oral administration of Sophora tonkinensis extract in rats.