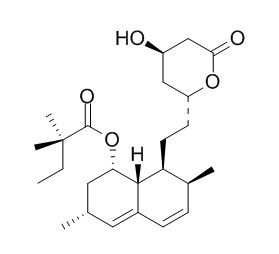
Simvastatin
Simvastatin is an FDA-approved cholesterol-lowering medication, it possesses potent anti-inflammatory property and has potent benefits on endothelial and smooth muscle cell-mediated vasomotor responses. Simvastatin rescues Aβ-mediated cerebrovascular and cognitive deficits. Simvastatin reduces burn-induced splenic apoptosis via downregulation of the TNF-α/NF-κB pathway, it induces p65 instability leads to MMP-9 down-regulation in leukemia cells, while it induces JNK1/c-Jun/ATF-2 activation maintains the MMP-2 expression underlying p65 down-regulation.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Sci Rep.2025, 15(1):29590.
Nutrients.2023, 15(12):2810.
Pak J Pharm Sci.2018, 31:311-315
Trop J Nat Prod Res, February2023, 7(2):2371-2381
Asian Pac J Tropical Bio.2020, 10(6):239-247
Int Immunopharmacol.2022, 106:108603.
Kor. J. Herbol.2022, 37(5): 89-96.
Food Funct.2022, 13(23):12105-12120.
J Med Food.2021, 24(2):151-160.
Processes2022, 10(10), 2008.
Related and Featured Products
J Cereb Blood Flow Metab. 2015 Mar;35(3):512-20.
Simvastatin restored vascular reactivity, endothelial function and reduced string vessel pathology in a mouse model of cerebrovascular disease.[Pubmed:
25564230]
Cerebrovascular dysfunction seen in Alzheimer's disease (AD) and vascular dementia (VaD) is multifaceted and not limited to the amyloid-β (Aβ) pathology. It encompasses structural alterations in the vessel wall, degenerating capillaries (string vessels), vascular fibrosis and calcification, features recapitulated in transgenic mice that overexpress transforming growth factor-β1 (TGF mice). We recently found that Simvastatin rescued Aβ-mediated cerebrovascular and cognitive deficits in a transgenic mouse model of AD. However, whether Simvastatin can counteract Aβ-independent deficits remains unknown.
METHODS AND RESULTS:
Here, we evaluated the effects of Simvastatin in aged TGF mice on cerebrovascular reactivity and structure, and on cognitive performance. Simvastatin restored baseline levels of nitric oxide (NO), NO-, and KATP channel-mediated dilations and endothelin-1-induced contractions. Simvastatin significantly reduced vasculopathy with arteriogenic remodeling and string vessel pathology in TGF mice. In contrast, Simvastatin did not lessen gliosis, and the cerebrovascular levels of pro-fibrotic proteins and calcification markers remained elevated after treatment. The TGF mice displayed subtle cognitive decline that was not affected by Simvastatin.
CONCLUSIONS:
Our results show potent benefits of Simvastatin on endothelial- and smooth muscle cell-mediated vasomotor responses, endothelial NO synthesis and in preserving capillary integrity. We conclude that Simvastatin could be indicated in the treatment of cerebrovascular dysfunction associated with VaD and AD.
J Invest Dermatol. 2015 Apr;135(4):1080-8.
Simvastatin prevents and reverses depigmentation in a mouse model of vitiligo.[Pubmed:
25521459]
Vitiligo is a common autoimmune disease of the skin that results in disfiguring white spots. There are no Food and Drug Administration (FDA)-approved treatments, and current treatments are time-consuming, expensive, and of low efficacy. We sought to identify new treatments for vitiligo, and first considered repurposed medications because of the availability of safety data and expedited regulatory approval. We previously reported that the IFN-γ-induced chemokine CXCL10 is expressed in lesional skin from vitiligo patients, and that it is critical for the progression and maintenance of depigmentation in our mouse model of vitiligo. We hypothesized that targeting IFN-γ signaling might be an effective new treatment strategy. Activation of signal transducer and activator of transcription 1 (STAT1) is required for IFN-γ signaling and recent studies revealed that Simvastatin, an FDA-approved cholesterol-lowering medication, inhibited STAT1 activation in vitro.
METHODS AND RESULTS:
Therefore, we hypothesized that Simvastatin may be an effective treatment for vitiligo. We found that Simvastatin both prevented and reversed depigmentation in our mouse model of vitiligo, and reduced the number of infiltrating autoreactive CD8(+) T cells in the skin. Treatment of melanocyte-specific, CD8(+) T cells in vitro decreased proliferation and IFN-γ production, suggesting additional effects of Simvastatin directly on T cells.
CONCLUSIONS:
Based on these data, Simvastatin may be a safe, targeted treatment option for patients with vitiligo.
Biochem Pharmacol. 2014 Dec 15;92(4):530-43.
Simvastatin induces NFκB/p65 down-regulation and JNK1/c-Jun/ATF-2 activation, leading to matrix metalloproteinase-9 (MMP-9) but not MMP-2 down-regulation in human leukemia cells.[Pubmed:
25316568]
The aim of the present study was to explore the signaling pathways associated with the effect of Simvastatin on matrix metalloproteinase-2 (MMP-2)/MMP-9 expression in human leukemia K562 cells.
CONCLUSIONS:
In sharp contrast to its insignificant effect on MMP-2, Simvastatin down-regulated MMP-9 protein expression and mRNA levels in K562 cells. Simvastatin-induced Pin1 down-regulation evoked NFκB/p65 degradation. Meanwhile, Simvastatin induced JNK-mediated c-Jun and ATF-2 activation. Over-expression of Pin1 suppressed Simvastatin-induced MMP-9 down-regulation. Treatment with SP600125 (a JNK inhibitor) or knock-down of JNK1 reduced MMP-2 expression in Simvastatin-treated cells. Simvastatin enhanced the binding of c-Jun/ATF-2 with the MMP-2 promoter. Down-regulation of c-Jun or ATF-2 by siRNA revealed that c-Jun/ATF-2 activation was crucial for MMP-2 expression. Suppression of p65 activation or knock-down of Pin1 by shRNA reduced MMP-2 and MMP-9 expression in K562 cells. Over-expression of constitutively active JNK1 rescued MMP-2 expression in Pin1 shRNA-transfected cells. Simvastatin treatment also suppressed MMP-9 but not MMP-2 expression in human leukemia U937 and KU812 cells.
CONCLUSIONS:
Taken together, our data indicate that Simvastatin-induced p65 instability leads to MMP-9 down-regulation in leukemia cells, while Simvastatin-induced JNK1/c-Jun/ATF-2 activation maintains the MMP-2 expression underlying p65 down-regulation.
Ann Surg. 2015 May;261(5):1006-12.
Simvastatin reduces burn injury-induced splenic apoptosis via downregulation of the TNF-α/NF-κB pathway.[Pubmed:
24950285 ]
Recent studies have suggested that epidermal burn injuries are associated with inflammation and immune dysfunction. Simvastatin has been shown to possess potent anti-inflammatory properties. Thus, we hypothesized that Simvastatin protects against burn-induced apoptosis in the spleen via its anti-inflammatory activity.
METHODS AND RESULTS:
Wild-type, tumor necrosis factor alpha knockout (TNF-α KO) and NF-κB KO mice were subjected to full-thickness burn injury or sham treatment. The mice then were treated with or without Simvastatin, and the spleen was harvested to measure the extent of apoptosis. Expression levels of TNF-α and NF-κB were also determined in spleen tissue and serum.
Burn injury induced significant splenic apoptosis and systemic cytokine production. Simvastatin protected the spleen from apoptosis, reduced cytokine production in the serum, and increased the survival rate. Simvastatin decreased burn-induced TNF-α and NF-κB expression in the spleen and serum. TNF-α and NF-κB KO mice demonstrated lower levels of apoptosis in spleen in response to burn injury. Simvastatin did not further decrease burn-caused apoptosis and mortality in either strain of KO mice.
CONCLUSIONS:
Simvastatin reduces burn-induced splenic apoptosis via downregulation of the TNF-α/NF-κB pathway.