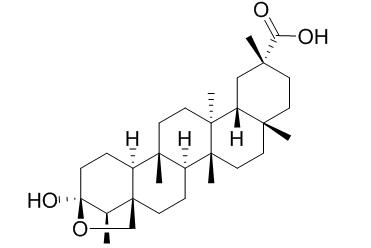
Salaspermic acid
Salaspermic acid is an inhibitor of HIV reverse transcriptase and HIV replication in H9 lymphocyte cells.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Natural Product Sciences2024, 30(1):8-13.
Plants (Basel).2021, 10(5):951.
Korean J of Pharmacognosy2020, 51,49-54.
Molecules.2017, 22(11)
University of Burgos2024, ssrn.4795441.
Appl. Sci.2021, 11(19),9343.
J Korean Soc Food Sci Nutr2023, 52(7): 750-757
J Agric Food Chem.2023, 71(47):18510-18523.
Molecules.2023, 28(8):3376.
Phytochemistry.2021, 181:112539.
Related and Featured Products
J Nat Prod. 1994 Jan;57(1):1-8.
Quinone-methide triterpenes and salaspermic acid from Kokoona ochracea.[Pubmed:
8158155]
METHODS AND RESULTS:
Tingenone[1],20-hydroxy-20-epi-tingenone[2],celastrol[ 3], and Salaspermic acid [4] have been isolated from Kokoona ochracea stem bark. The quinone-methide triterpenes 1-3 exhibited strong but non-specific in vitro cytotoxicity against P-388 murine lymphocytic leukemia cells and a panel of human cancer cell lines. Salaspermic acid [4] was not active in all the cancer cell lines used in this investigation.
CONCLUSIONS:
13C-nmr spectra assignments for Salaspermic acid have been accomplished through the application of 1D and 2D nmr spectral techniques, and 13C-nmr assignments for celastrol [3] have been revised.
J Nat Prod. 1992 Mar;55(3):340-6.
Anti-aids agents, 6. Salaspermic acid, an anti-HIV principle from Tripterygium wilfordii, and the structure-activity correlation with its related compounds.[Pubmed:
1375626]
METHODS AND RESULTS:
Salaspermic acid [1], an inhibitor of HIV reverse transcriptase and HIV replication in H9 lymphocyte cells, was isolated from the roots of Tripterygium wilfordii for the first time. The structure of 1 derived from spectral data was established unequivocally by an X-ray analysis of crystals of the monohydrate.
CONCLUSIONS:
A structure-activity correlation of 1 with ten related compounds indicated that the acetal linkage in ring A and the carboxyl group in ring E of 1 may be required for the anti-HIV activity.