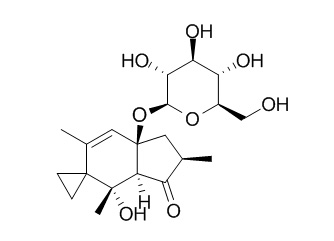
Ptaquiloside
Ptaquiloside shows genotoxicity, it also has carcinogenic effects. Ptaquiloside has immunosuppressive effects, it reduces NK cell activities by enhancing metallothionein expression, which is prevented by selenium.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Eur J Pharmacol.2021, 906:174220.
Phytother Res.2022, 10.1002:ptr.7602.
J Agric Food Chem.2024, acs.jafc.4c01387.
Int J Mol Sci.2019, 20(11):E2734
Applied Biological Chemistry2022, 71:s13765-022-00743-5.
Foods.2022, 12(1):136.
Evid Based Complement Alternat Med.2018, 2018:8565132
J Chromatogr Sci.2015, 53(5):824-9
Chinese Pharmaceutical Journal2023, 58(2):178-187.
Clin Exp Pharmacol Physiol.2020, doi: 10.1111
Related and Featured Products
Environ Toxicol Chem. 2005 Nov;24(11):2751-6.
Genotoxic activity and inhibition of soil respiration by ptaquiloside, a bracken fern carcinogen.[Pubmed:
16398109]
Ptaquiloside (PTA) is a natural toxin produced by bracken (Pteridium aquilinum [L.] Kuhn). Assessment of PTA toxicity is needed because PTA deposited from bracken to soil may leach to surface and groundwater.
METHODS AND RESULTS:
Inhibition of soil respiration and genotoxic activity of PTA was determined by a soil microbial carbon transformation test and an umu test, respectively. In the carbon transformation test, sandy loam soil was incubated at five different initial concentrations of PTA for a period of 28 d, after which glucose was added and respiration measured for 12 consecutive hours. The tests were performed at 20 degrees C and soil moisture content of approximately 15%. For soil material sampled in the autumn, initial PTA concentrations ranging from 0.008 to 40.6 microg PTA/g dry soil were tested. From fitting of data by a sigmoidal function, a 10% effect dose (ED10) was estimated to 13 microg PTA/ g dry soil, with an upper 95% confidence limit of 43 microg PTA/g dry soil and a 95% lower confidence limit of -infinity microg PTA/g dry soil. For soil material sampled in late winter, initial PTA concentrations ranging from 1.56 to 212 microg PTA/g dry soil were tested, resulting in an ED10 value of 55 microg PTA/g dry soil, with an upper 95% confidence limit of 70 microg PTA/g dry soil and a 95% lower confidence limit of 40 microg PTA/g dry soil. The genotoxic activity of PTA was determined using the umu test without and with metabolic activation (addition of S9 rat liver homogenate). In tests with addition of S9, the induction ratio exceeded the critical ratio of 1.5 at a PTA concentration of 46 +/- 16 microg/ml and, in tests without S9, the critical ratio was exceeded at a PTA concentration of 279 +/- 22 microg/ml.
CONCLUSIONS:
The genotoxicity of PTA is comparable to that of quercetin, another bracken constituent. The toxicity of PTA toward microorganisms prolongs the persistence of PTA in terrestrial environments, increasing the risk of PTA leaching to drainage and groundwater.
Toxicology. 2013 Feb 8;304:100-8.
Ptaquiloside reduces NK cell activities by enhancing metallothionein expression, which is prevented by selenium.[Pubmed:
23274088 ]
Pteridium aquilinum, one of the most important poisonous plants in the world, is known to be carcinogenic to animals and humans. Moreover, our previous studies showed that the immunosuppressive effects of Ptaquiloside, its main toxic agent, were prevented by selenium in mouse natural killer (NK) cells. We also verified that this immunosuppression facilitated development of cancer.
METHODS AND RESULTS:
Here, we performed gene expression microarray analysis in splenic NK cells from mice treated for 14 days with Ptaquiloside (5.3 mg/kg) and/or selenium (1.3 mg/kg) to identify gene transcripts altered by Ptaquiloside that could be linked to the immunosuppression and that would be prevented by selenium. Transcriptome analysis of Ptaquiloside samples revealed that 872 transcripts were expressed differentially (fold change>2 and p<0.05), including 77 up-regulated and 795 down-regulated transcripts. Gene ontology analysis mapped these up-regulated transcripts to three main biological processes (cellular ion homeostasis, negative regulation of apoptosis and regulation of transcription). Considering the immunosuppressive effect of Ptaquiloside, we hypothesized that two genes involved in cellular ion homeostasis, metallothionein 1 (Mt1) and metallothionein 2 (Mt2), could be implicated because Mt1 and Mt2 are responsible for zinc homeostasis, and a reduction of free intracellular zinc impairs NK functions. We confirm these hypotheses and show increased expression of metallothionein in splenic NK cells and reduction in free intracellular zinc following treatment with Ptaquiloside that were completely prevented by selenium co-treatment.
CONCLUSIONS:
These findings could help avoid the higher susceptibility to cancer that is induced by P. aquilinum-mediated immunosuppressive effects.
Br J Cancer. 2000 Oct;83(7):914-20.
Carcinogenic effects of ptaquiloside in bracken fern and related compounds.[Pubmed:
10970694 ]
Consumption of the bracken fern Pteridium aquilinum by cattle has been shown to induce bladder and intestinal carcinomas in cattle and to cause a number of diseases in other farm animals. An unstable glucoside named Ptaquiloside, containing a reactive cyclopropane ring, has been isolated from the fern and its potent carcinogenicity proven.
METHODS AND RESULTS:
Nineteen of 31 ferns tested by chemotaxonomic methods in Japan have been found to contain potentially carcinogenic Ptaquilosides as have Cheilanthes sieberi and Pteridium esculentum. Hydrolysis of Ptaquilosides leads to pterosins; under milder conditions a dienone which is believed to be the primary carcinogen is obtained. Hypacrone, a sesquiterpine containing a reactive cyclopropane ring, has been isolated from Hypolepis punctata and its structure proved by synthesis. Illudins, structurally similar to Ptaquiloside, have been isolated from the basidiomycete Omphalotus illudens. These give anti-tumour activity and similar reactivity with nucleophiles to Ptaquiloside. Compound CC-1065, a highly toxic antibiotic also containing a cyclopropane ring, has been isolated from Streptomyces zelensis. The mechanism of its reactivity with DNA has been compared to that of Ptaquiloside and the small structural differences between carcinogenic and anti-tumour activity discussed.
CONCLUSIONS:
Both CC-1065 and adozelesin, a synthetic analogue with anti-tumour activity, have been shown to alkylate the N-3 atom of adenine in a certain sequence of DNA. The reactivity of cysteine with Ptaquilosides and illudins is discussed, as is the role of cysteine alkylating agents in apoptosis.