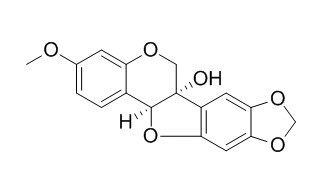
Pisatin
Pisatin is an isoflavonoid phytoalexin synthesized by pea (Pisum sativum L.).
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Curr Issues Mol Biol.2024, 46(4):3328-3341.
Molecules.2020, 25(18):4283.
Food Funct.2024, 15(8):4262-4275.
Analytical Letters.2020, doi 10.1008
Anat Rec (Hoboken).2021, 304(2):323-332.
J Appl Biol Chem.2021, 64(3),263?268
Br J Pharmacol.2024, 181(24):5009-5027.
Horticulturae2024, 10(5), 486.
Vojnosanit Pregl2016, 75(00):391-391
J Agric Food Chem.2024, acs.jafc.4c01387.
Related and Featured Products
Molecules. 2014 Dec 23;20(1):24-34.
EDTA a novel inducer of pisatin, a phytoalexin indicator of the non-host resistance in peas.[Pubmed:
25546618]
Pea pod endocarp suppresses the growth of an inappropriate fungus or non-pathogen by generating a "non-host resistance response" that completely suppresses growth of the challenging fungus within 6 h. Most of the components of this resistance response including Pisatin production can be elicited by an extensive number of both biotic and abiotic inducers. Thus this phytoalexin serves as an indicator to be used in evaluating the chemical properties of inducers that can initiate the resistance response. Many of the Pisatin inducers are reported to interact with DNA and potentially cause DNA damage.
METHODS AND RESULTS:
Here we propose that EDTA (ethylenediaminetetraacetic acid) is an elicitor to evoke non-host resistance in plants. EDTA is manufactured as a chelating agent, however at low concentration it is a strong elicitor, inducing the phytoalexin Pisatin, cellular DNA damage and defense-responsive genes. It is capable of activating complete resistance in peas against a pea pathogen. Since there is also an accompanying fragmentation of pea DNA and alteration in the size of pea nuclei, the potential biochemical insult as a metal chelator may not be its primary action.
CONCLUSIONS:
The potential effects of EDTA on the structure of DNA within pea chromatin may assist the transcription of plant defense genes.
Mol Plant Microbe Interact. 2004 Jul;17(7):798-804.
Introduction of plant and fungal genes into pea (Pisum sativum L.) hairy roots reduces their ability to produce pisatin and affects their response to a fungal pathogen.[Pubmed:
15242174]
Pisatin is an isoflavonoid phytoalexin synthesized by pea (Pisum sativum L.). Previous studies have identified two enzymes apparently involved in the synthesis of this phytoalexin, isoflavone reductase (IFR), which catalyzes an intermediate step in Pisatin biosynthesis, and (+)6a-hydroxymaackiain 3-O-methyltransferase (HMM), an enzyme catalyzing the terminal step.
METHODS AND RESULTS:
To further evaluate the involvement of these enzymes in Pisatin biosynthesis, sense- and antisense-oriented cDNAs of Ifr and Hmm fused to the 35s CaMV promoter, and Agrobacterium rhizogenes, were used to produce transgenic pea hairy root cultures. PDA, a gene encoding Pisatin demethylating activity (pda) in the pea-pathogenic fungus Nectria haematococca, also was used in an attempt to reduce Pisatin levels. Although hairy root tissue with either sense or antisense Ifr cDNA produced less Pisatin, the greatest reduction occurred with sense or antisense Hmm cDNA. The reduced Pisatin production in these lines was associated with reduced amounts of Hmm transcripts, HMM protein, and HMM enzyme activity. Hairy roots containing the PDA gene also produced less Pisatin. To evaluate the role of Pisatin in disease resistance, the virulence of N. haematococca on the transgenic roots that produced the lowest levels of Pisatin was tested.
CONCLUSIONS:
Hairy roots expressing antisense Hmm were more susceptible than the control hairy roots to isolates of N. haematococca that are either virulent or nonvirulent on wild-type pea plants. This appears to be the first case of producing transgenic plant tissue with a reduced ability to produce a phytoalexin and demonstrating that such tissue is less resistant to fungal infection: these results support the hypothesis that phytoalexin production is a disease resistance mechanism.
Fungal Genet Biol. 2001 Jun;33(1):37-48.
Characterization of pisatin-inducible cytochrome p450s in fungal pathogens of pea that detoxify the pea phytoalexin pisatin.[Pubmed:
11407884]
Many fungi that are pathogenic on pea have the ability to demethylate and thus detoxify the pea phytoalexin Pisatin. This detoxification reaction has been studied most thoroughly in Nectria haematococca MP VI where it functions as a virulence trait. The enzyme catalyzing this reaction [Pisatin demethylase (pda)] is a cytochrome P450.
METHODS AND RESULTS:
In the current study, the induction of whole-cell pda activity and the biochemical properties of pda in microsomal preparations from the pea pathogens Ascochyta pisi, Mycosphaerella pinodes, and Phoma pinodella are compared to the pda produced by N. haematococca. Based on cofactor requirements and their inhibition by carbon monoxide, cytochrome P450 inhibitors, and antibodies to NADPH:cytochrome P450 reductase, we conclude that the pdas from the other pea pathogens also are cytochrome P450s.
CONCLUSIONS:
All of the enzymes show a rather selective induction by Pisatin, have a low K(m) toward Pisatin, and have a fairly high degree of specificity toward Pisatin as a substrate, suggesting that each pathogen may have a specific cytochrome P450 for detoxifying this plant antibiotic.
Since the pdas in these fungi differ in their pattern of sensitivity to P450 inhibitors and display other minor biochemical differences, we suggest that these fungi may have independently evolved a specialized cytochrome P450 as a virulence trait for a common host.