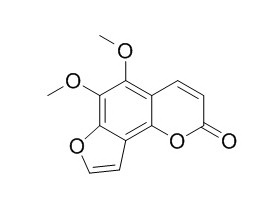
Pimpinellin
Pimpinellin has phototoxic, it acts as antagonist of proteins with GABA receptor activity. Pimpinellin has anti- inflammatory, and cytotoxic activities. Pimpinellin also has anti-bacterial activity, it shows inhibitory activity against Mycobacterium tuberculosis strain H37Ra , with MICs of 812 uM and IC50s of 389 uM.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Molecules.2019, 24(2):329
Int. J. Mol. Sci.2022, 23(14),7699;
Korea Food Research Institute2024, 4798082
Arch Biochem Biophys.2020, 687:108363.
Pharmacol Res.2022, 182:106346.
Molecules.2019, 24(22):E4022
University of Limpopo2016, 1777
Evid Based Complement Alternat Med.2021, 8855980.
Antioxidants (Basel).2020, 9(6):544.
Pathol Res Pract.2024, :260:155445.
Related and Featured Products
J Ethnopharmacol. 2013 May 2;147(1):232-7.
The Canadian medicinal plant Heracleum maximum contains antimycobacterial diynes and furanocoumarins.[Pubmed:
23501157]
Heracleum maximum is amongst the most commonly used plants by the indigenous peoples of North America. The First Nations of the eastern Canada use infusions of Heracleum maximum roots for the treatment of respiratory ailments including tuberculosis. Previous investigations of extracts derived from the roots of Heracleum maximum have shown it to possess antimycobacterial activity.
To isolate and identify antimycobacterial constituents from the roots of Heracleum maximum.
METHODS AND RESULTS:
A methanolic extract of Heracleum maximum roots was subjected to bioassay guided fractionation using the microplate resazurin assay (MRA) to assess inhibitory activity against Mycobacterium tuberculosis strain H37Ra. The antimycobacterial constituents were identified by NMR, MS and polarimetry.
The polyacetylene (3R,8S)-falcarindiol and the furanocoumarins bergapten, isobergapten, angelicin, sphondin, Pimpinellin, isoPimpinellin and 6-isopentenyloxyisobergapten were isolated from the Heracleum maximum root extract. (3R,8S)-Falcarindiol and 6-isopentenyloxyisobergapten exhibited MICs of 24 μM and 167 μM and IC50s of 6 μM and 27 μM against Mycobacterium tuberculosis H37Ra respectively. The remaining furanocoumarins bergapten, isobergapten, angelicin, sphondin, Pimpinellin, and isoPimpinellin were less active, with MICs of 925, 1850, 2149, 1859, 812 and 1625 μM and IC50s of 125, 344, 350, 351, 389 and 406 μM.
CONCLUSIONS:
(3R,8S)-Falcarindiol, bergapten, isobergapten, angelicin, sphondin, Pimpinellin, isoPimpinellin and 6-isopentenyloxyisobergapten were identified as the principal constituents responsible for the antimycobacterial activity of the roots of Heracleum maximum. This work supports the ethnopharmacological use of Heracleum maximum by Canadian First Nations and Native American communities as a treatment for infectious diseases, specifically tuberculosis.
J Chromatogr B Analyt Technol Biomed Life Sci. 2012 Apr 1;891-892:102-8.
Simultaneous determination of pimpinellin, isopimpinellin and phellopterin in rat plasma by a validated UPLC-MS/MS and its application to a pharmacokinetic study after administration of Toddalia asiatica extract.[Pubmed:
22418072]
METHODS AND RESULTS:
A rapid and selective ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method was developed for simultaneous determination of three bioactive coumarins of Toddalia asiatica extract including Pimpinellin, isoPimpinellin and phellopterin in rat plasma for the first time. Phenacetin was used as the internal standard (IS). Plasma samples were extracted by liquid-liquid extraction with methyl tert-butyl ether. The chromatographic separation was carried out on an ACQUITY UPLC™ BEH C₁₈ column with an isocratic mobile phase consisting of methanol-5 mmol/L ammonium acetate (65:35, v/v). The detection was performed on a triple quadrupole tandem mass spectrometer by multiple reaction monitoring (MRM) via electrospray ionization (ESI) source with positive ionization mode. The method was linear for all analytes over investigated range with all correlation coefficients greater than 0.9942. The lower limits of quantification (LLOQ) were 25.0 ng/mL for Pimpinellin, 10.0 ng/mL for isoPimpinellin and 5.00 ng/mL for phellopterin. The intra- and inter-day precision (RSD%) was within 12% and the accuracy (RE%) ranged from -2.3% to 5.5%.
CONCLUSIONS:
The rapid and sensitive method was fully validated and successfully applied to the pharmacokinetic study of Pimpinellin, isoPimpinellin and phellopterin in rats following oral administration of Toddalia asiatica extract.
Chem Cent J. 2013 Feb 4;7(1):24.
Discovery and antitumor activities of constituents from Cyrtomium fortumei (J.) Smith rhizomes.[Pubmed:
23379693 ]
Cyrtomium fortumei (J.) Smith is an important Chinese herbal medicine because of its biological functions. However, systematic and comprehensive studies on the phytochemicals from Cyrtomium fortumei (J.) Smith and their bioactivity are limited.
METHODS AND RESULTS:
Using the bioassay-guided technique, the ethyl acetate and n-BuOH extracts of the rhizomes of Cyrtomium fortumei (J.) Smith were shown to exhibit good antitumor activities, consequently leading to the isolation of 23 compounds. All compounds were isolated from the plant for the first time. The inhibitory activities of these compounds were investigated on tumor cells MGC-803, PC3, and A375 in vitro by MTT (thiazolyl blue tetrazolium bromide) assay, and the results showed that Pimpinellin (3) had potent cytotoxic activities against the three cell lines, with the IC50 values of 14.4 ± 0.3 μM, 20.4 ± 0.5 μM, and 29.2 ± 0.6 μM, respectively. The mechanism of the antitumor action indicated that Pimpinellin inhibited the growth of MGC-803 cells via the induction of tumor cell apoptosis, with apoptosis ratio of 27.44% after 72 h of treatment at 20 μM.
CONCLUSIONS:
This study suggests that most of the compounds from the roots of Cyrtomium fortumei (J.) Smith could inhibit the growth of human carcinoma cells. Moreover, Pimpinellin inhibited the growth of tumor cells via the induction of tumor cell apoptosis.
Contact Dermatitis. 1983 Sep;9(5):364-6.
Phototoxicity from furocoumarins (psoralens) of Heracleum laciniatum in a patient with vitiligo. Action spectrum studies on bergapten, pimpinellin, angelicin and sphondin.[Pubmed:
6627920]
Investigations on light reactions in a patient with vitiligo are presented.
METHODS AND RESULTS:
The minimal erythema dose (MED) in the UVB area was approximately 1/3 of that in persons of skin type II. The application of furocoumarins (psoralens) increased light tolerance by 1 MED at 300-310 nm. Action spectrum studies with furocoumarins from Heracleum laciniatum showed the following order of potency: bergapten, Pimpinellin, angelicin and sphondin.
CONCLUSIONS:
The efficacy was highest at 325-350 nm, with maxima at 330-335 nm. Pimpinellin was recently found to be phototoxic, but an action spectrum of sphondin is reported for the first time.