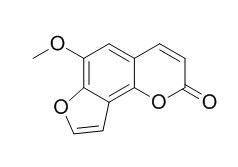
Sphondin
Sphondin may have anticonvulsant, anti-inflammatory, and anti-proliferative activities, it may act as a potent inhibitor of NO production under tissue-damaging inflammatory conditions, it also
possesses an inhibitory effect on IL-1beta-induced increase in the level of COX-2 protein and PGE(2) release in A549 cells through suppression of NF-kappaB activity. Sphondin, 8-methoxypsoralen, and khellin have delayed phototoxic effects inAedes aegypti.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Biomed Pharmacother.2019, 116:108987
Processes2021, 9(1), 153.
Biomed Pharmacother.2024, 181:117647.
Anticancer Res.2018, 38(4):2127-2135
Phytochem Anal.2016, 27(5):296-303
Cells.2023, 12(1):168.
Molecules.2024, 29(5):1050.
JEJU National University2022, 10478.
Pharmaceuticals (Basel).2022, 15(8):982.
J Control Release.2024, 375:300-315.
Related and Featured Products
Contact Dermatitis. 1983 Sep;9(5):364-6.
Phototoxicity from furocoumarins (psoralens) of Heracleum laciniatum in a patient with vitiligo. Action spectrum studies on bergapten, pimpinellin, angelicin and sphondin.[Pubmed:
6627920 ]
Investigations on light reactions in a patient with vitiligo are presented.
METHODS AND RESULTS:
The minimal erythema dose (MED) in the UVB area was approximately 1/3 of that in persons of skin type II. The application of furocoumarins (psoralens) increased light tolerance by 1 MED at 300-310 nm. Action spectrum studies with furocoumarins from Heracleum laciniatum showed the following order of potency: bergapten, pimpinellin, angelicin and Sphondin. The efficacy was highest at 325-350 nm, with maxima at 330-335 nm.
CONCLUSIONS:
Pimpinellin was recently found to be phototoxic, but an action spectrum of Sphondin is reported for the first time.
J Nat Med. 2014 Jan;68(1):83-94.
Anti-tumor effects of various furocoumarins isolated from the roots, seeds and fruits of Angelica and Cnidium species under ultraviolet A irradiation.[Pubmed:
23649674]
METHODS AND RESULTS:
We examined the effects on cell proliferation of 10 methoxyfurocoumarins and 7 dihydrofurocumarins isolated from Umbelliferae medicinal plants, and their mechanisms of action against B16F10 melanoma cells or in melanin-possessing hairless mice implanted with B16F10 melanoma cells, under UVA irradiation. Furocoumarins having a methoxy group, such as bergapten (1), xanthotoxin (2), phellopterin (4), byakangelicin (6), neobyakangelicin (8), isobergapten (9) and Sphondin (10), showed anti-proliferative activity and caused G2/M arrest at concentrations of 0.05-15.0 μM. The 7 dihydrofurocoumarins had no effect. UVA plus 1, 2, 4, 6 and sec-O-acetylbyakagelicin (7), having one methoxy group at the C-5 position and a linear-type conformation, reduced tumor growth and final tumor weight in B16F10-bearing mice at 0.5 or 1.0 mg/kg (intraperitoneal injection). UVA plus 1 and 2 increased Chk1 phosphorylation and decreased cdc2 (Thr 161) phosphorylation in the melanoma cells.
CONCLUSIONS:
The anti-tumor actions of UVA plus furocoumarins having a methoxy group might be due to the arrest of the cell cycle at G2/M through an increase in phospho-Chk1 and reduction in phospho-cdc2.
Food Chem., 2008, 107(3):990-3.
Anticonvulsant activity of furanocoumarins and the essential oil obtained from the fruits of Heracleum crenatifolium.[Reference:
WebLink]
The anticonvulsant activity of furanocoumarins, coumarin mixture and the essential oil obtained from the fruits of Heracleum crenatifolium was examined against maximal electroshock (MES)-induced seizures in mice.
METHODS AND RESULTS:
Bergapten showed significant anticonvulsant activity. The furanocoumarins isolated from the fruits of the plant were identified using thin-layer chromatography, melting points and spectroscopic methods (IR, MS, 1H NMR) as isobergapten (1), pimpinellin (2), bergapten (3), isopimpinellin (4), Sphondin (5) and byak-angelicol (6). The essential oil content of the fruits were found as 5.5%.
Twenty-two compounds representing 99.3% of the essential oil obtained from the fruits of H. crenatifolium were determined and the major components were identified as octanol and octyl acetate (3.1% and 88.4% respectively) by GC and GC–MS.
Life Sci. 2002 Nov 29;72(2):199-213.
Effects of sphondin, isolated from Heracleum laciniatum, on IL-1beta-induced cyclooxygenase-2 expression in human pulmonary epithelial cells.[Pubmed:
12417253]
Recently, under large-scale screening experiments, we found that Sphondin, a furanocoumarin derivative isolated from Heracleum laciniatum, possessed an inhibitory effect on IL-1beta-induced increase in the level of COX-2 protein and PGE(2) release in A549 cells. Accordingly, we examined in the present study the action mechanism of Sphondin on the inhibition of IL-1beta-induced COX-2 protein expression and PGE(2) release in a human pulmonary epithelial cell line (A549).
METHODS AND RESULTS:
Pretreatment of cells with Sphondin (10-50 microM) concentration-dependently attenuated IL-1beta-induced COX-2 protein expression and PGE(2) release. The IL-1beta-induced increase in COX-2 mRNA expression was also attenuated by Sphondin (50 microM). The selective COX-2 inhibitor, NS-398 (0.01-1 microM), inhibited the activity of the COX-2 enzyme in a concentration-dependent manner, while Sphondin (10-50 microM) had no effect. Sphondin (50 microM) did not affect the IL-1beta-induced activations of p44/42 MAPK, p38 MAPK, and JNK. Treatment of cells with Sphondin (50 microM) or the NF-kappaB inhibitor, PDTC (50 microM) partially inhibited IL-1beta-induced degradation of IkappaB-alpha in the cytosol and translocation of p65 NF-kappaB from the cytosol to the nucleus. Furthermore, IL-1beta-induced NF-kappaB-specific DNA-protein complex formation in the nucleus was partially inhibited by Sphondin (50 microM) or PDTC (50 microM).
CONCLUSIONS:
Taken together, we demonstrate that Sphondin inhibits IL-1beta-induced PGE(2) release in A549 cells; this inhibition is mediated by suppressing of COX-2 expression, rather than by inhibiting COX-2 enzyme activity. The inhibitory mechanism of Sphondin on IL-1beta-induced COX-2 expression may be, at least in part, through suppression of NF-kappaB activity. We conclude that Sphondin may have the therapeutic potential as an anti-inflammatory drug on airway inflammation.
Bioorg Med Chem. 2000 Dec;8(12):2701-7.
Inducible nitric oxide synthase inhibitors of Chinese herbs. Part 2: naturally occurring furanocoumarins.[Pubmed:
11131161]
Inducible nitric oxide synthase (iNOS)-dependent production of nitric oxide (NO) plays an important role in inflammation.
METHODS AND RESULTS:
The effects of various naturally occurring furanocoumarins on NO production in lipopolysaccharide (LPS)-activated RAW 264.7 macrophage cells were evaluated in vitro. The results showed that angelicin, pimpinellin, Sphondin, byakangelicol, oxypeucedanin, oxypeucedanin hydrate, xanthotoxin, and cnidilin are potential NO production inhibitors, and their IC50 values for inhibition of nitrite production were 19.5, 15.6, 9.8, 16.9, 16.8, 15.8, 16.6, and 17.7 microg/mL, respectively. Distinct structure-activity relationships were also revealed for the NO production inhibitory activities of these furanocoumarins. Activities of the angelicin type such as pimpinellin and Sphondin were more potent than those of the psoralen type. Presence of a methoxy at the C6 position in the angelicin type seemed to be essential to augment the activity. Western blot analysis demonstrated that only Sphondin dose-dependently inhibited the expression of the iNOS protein at 2.5-20 microg/mL. However, iNOS enzyme activity was stimulated with LPS for 12 h and Sphondin was administered (20 microg/mL) for 24 h, which did not reasonably inhibit iNOS enzyme activity. L-NAME (100 microM), a known specific inhibitor of iNOS, was employed as a positive control with the same protocol and showed more than 50% inhibition activity.
CONCLUSIONS:
The results demonstrate that the NO production inhibitory activity of Sphondin is due to the effect of iNOS expression, but not by direct inhibition of iNOS enzyme activity. Thus, Sphondin may act as a potent inhibitor of NO production under tissue-damaging inflammatory conditions.
J Chem Ecol. 1986 Apr;12(4):899-914.
Delayed phototoxic effects of 8-methoxypsoralen, khellin, and sphondin inAedes aegypti.[Pubmed:
24306978 ]
METHODS AND RESULTS:
At concentrations up to 6.7 ppm, 8-methoxypsoralen, Sphondin, and khellin are not toxic to first-instar larvae of the mosquitoAedes aegypti. The irradiation of sensitized larvae with long-wavelength ultraviolet light did not always produce any immediate toxicity enhancement, but delayed effects were clearly visible. These were observed over the development of the organisms from first-instar larvae to adults. No adverse effects were noted when larvae were irradiated in the absence of sensitizers, or when they were placed in solutions of sensitizers which had been previously irradiated with the same light sources.
CONCLUSIONS:
8-Methoxypsoralen was slightly more phototoxic than its isomer Sphondin. Khellin, recently reported to undergo photoinduced cyclization with DNA components, showed minimal phototoxicity in the concentration range used.