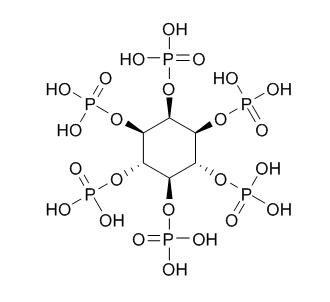
Phytic acid
Phytic acid is the principal storage form of phosphorus in many plant tissues, especially bran and seeds. It can act as a cofactor in DNA repair by nonhomologous end-joining. It is a trypsin inhibitor, has chelating, antioxidant, anti-inflammatory, and neuroprotective effects, it forms an iron chelate which greatly accelerates Fe2+-mediated oxygen reduction yet blocks iron-driven hydroxyl radical generation and suppresses lipid peroxidation. High concentrations of phytic acid prevent browning and putrefaction of various fruits and vegetables by inhibiting polyphenol oxidase, it may be a substitute for presently employed preservatives.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Curr Pharm Des.2024, 30(1):71-80.
BMC Complement Altern Med.2019, 19(1):11
Appl. Sci. 2021, 11(10),4666.
Food Science and Human Wellness2022, 11(4):965-974
Chinese Journal of Tissue Engineering Research2024, 28(8):1149-1154.
Molecules.2023, 28(8):3503.
J Ethnopharmacol.2017, 209:305-316
EXCLI J.2023, 22:482-498.
J Sci Food Agric.2023, 103(1):213-220.
Food Engineering Progress2019, 23(3)209-216
Related and Featured Products
J Mech Behav Biomed Mater. 2015 Apr 6;48:145-152.
Effect of phytic acid etchant on the structural stability of demineralized dentine and dentine bonding.[Pubmed:
25933170]
This study examined the effect of Phytic acid (IP6) in stabilizing the morphology of dentine collagen network and resin-dentine bonding.
METHODS AND RESULTS:
Dentine beams were fully demineralized with 10% phosphoric acid (PA) or 1% IP6 (pH 1.2). PA-demineralized beams were divided into three groups: (a) no further treatment (control), (b) treatment with 5% glutaraldehyde (GA) for 1 h and (c) treatment with 1% IP6 (pH 7) for 1 h. IP6-demineralized beams received no further treatment. The beams were then subjected to ultimate tensile strength (UTS) testing. Dentine micromorphology evaluation was performed using a field-emission scanning electron microscope (FE-SEM). Dentine disks were etched with 35% PA for 15 s or 1% IP6 for 30s. PA-etched dentine disks were divided into three groups as (a), (b) and (c) as for UTS testing, but the treatment with GA or IP6 was done in 1min. For microtensile bond strength (μTBS) testing, flat dentine surfaces etched with PA or IP6 were blot-dried (wet dentine) or air-dried for 10s (dry dentine) and bonded with an etch-and-rinse adhesive followed by composite build-up.
IP6-demineralized dentine showed significantly higher UTS, when compared to PA-demineralized dentine. GA and IP6 significantly improved UTS of PA-demineralized dentine. FE-SEM observation revealed that dentine collagen network was preserved by GA and IP6. No significant difference in μTBS was found between the wet and dry IP6-etched dentine groups.
CONCLUSIONS:
IP6 etching showed a structural stabilizing effect on demineralized dentine matrix and produced good resin-dentine bonding, regardless of dentine moistness or dryness.
Biosens Bioelectron. 2015 Mar 20;70:232-238.
METHODS AND RESULTS:
We herein report a facile, one-step pyrolysis synthesis of photoluminescent carbon dots (CDs) using citric acid as the carbon source and lysine as the surface passivation reagent. The as-prepared CDs show narrow size distribution, excellent blue fluorescence and good photo-stability and water dispersivity. The fluorescence of the CDs was found to be effectively quenched by ferric (Fe(III)) ions with high selectivity via a photo-induced electron transfer (PET) process. Upon addition of Phytic acid (PA) to the CDs/Fe(III) complex dispersion, the fluorescence of the CDs was significantly recovered, arising from the release of Fe(III) ions from the CDs/Fe(III) complex because PA has a higher affinity for Fe(III) ions compared to CDs. Furthermore, we developed an "off-on" fluorescence assay method for the detection of Phytic acid using CDs/Fe(III) as a fluorescent probe. This probe enables the selective detection of PA with a linear range of 0.68-18.69 μM and a limit of detection (signal-to-noise ratio is 3) of 0.36 μM.
CONCLUSIONS:
The assay method demonstrates high selectivity, repeatability, stability and recovery ratio in the detection of the standard and real PA samples. We believe that the facile operation, low-cost, high sensitivity and selectivity render this CD-based "off-on" fluorescent probe an ideal sensing platform for the detection of PA.
J. Biol. Chem., 1987, 262(24):11647-50.
Phytic acid. A natural antioxidant.[Reference:
WebLink]
METHODS AND RESULTS:
The catalysis by iron of radical formation and subsequent oxidative damage has been well documented. Although many iron-chelating agents potentiate reactive oxygen formation and lipid peroxidation, Phytic acid (abundant in edible legumes, cereals, and seeds) forms an iron chelate which greatly accelerates Fe2+-mediated oxygen reduction yet blocks iron-driven hydroxyl radical generation and suppresses lipid peroxidation. Furthermore, high concentrations of Phytic acid prevent browning and putrefaction of various fruits and vegetables by inhibiting polyphenol oxidase.
CONCLUSIONS:
These observations indicate an important antioxidant function for phytate in seeds during dormancy and suggest that phytate may be a substitute for presently employed preservatives, many of which pose potential health hazards.
Neurosci Lett. 2015 Apr 27;597:132-136.
Phytic acid attenuates inflammatory responses and the levels of NF-κB and p-ERK in MPTP-induced Parkinson's disease model of mice.[Pubmed:
25929185]
Phytic acid (PA) is a naturally occurring constituent which exhibits protective action in Parkinson's disease (PD). Inflammation in the central nervous system (CNS) is strongly associated with neuronal death in PD. However, the molecular mechanism of the protective effect of Phytic acid in PD has not been fully elucidated.
METHODS AND RESULTS:
In this study, we tried to testify the protection of Phytic acid on neuron and inflammatory responses in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD model of mice and investigated the mechanism involved in them. Motor behavior test and tyrosine hydroxylase (TH) immunohistochemistry method showed Phytic acid significantly inhibited MPTP-induced dopaminergic cell loss in the substantia nigra (SN). Moreover, using immunohistochemistry method and quantitative polymerase chain reaction (qPCR), microglial activation and inducible nitric oxide synthase (iNOS) were found to be markedly repressed by Phytic acid . Via western blot assay, expressions of nuclear factor κB (NF-κB) and phosphorylated extracellular signal-regulated kinase (p-ERK) were significantly attenuated by Phytic acid .
CONCLUSIONS:
In conclusion, it is suggested that Phytic acid has a neuroprotective effect in MPTP-induced PD model and the neuroprotection is correlated with its anti-inflammatory effect which may be associated with suppression of pathways that involved in NF-κB and p-ERK.
J Nutr. 1995 Mar;125(3 Suppl):581S-588S.
Compositional changes in trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing.[Pubmed:
7884537]
METHODS AND RESULTS:
We herein report a facile, one-step pyrolysis synthesis of photoluminescent carbon dots (CDs) using citric acid as the carbon source and lysine as the surface passivation reagent. The as-prepared CDs show narrow size distribution, excellent blue fluorescence and good photo-stability and water dispersivity. The fluorescence of the CDs was found to be effectively quenched by ferric (Fe(III)) ions with high selectivity via a photo-induced electron transfer (PET) process. Upon addition of Phytic acid (PA) to the CDs/Fe(III) complex dispersion, the fluorescence of the CDs was significantly recovered, arising from the release of Fe(III) ions from the CDs/Fe(III) complex because PA has a higher affinity for Fe(III) ions compared to CDs. Furthermore, we developed an "off-on" fluorescence assay method for the detection of Phytic acid using CDs/Fe(III) as a fluorescent probe. This probe enables the selective detection of PA with a linear range of 0.68-18.69 μM and a limit of detection (signal-to-noise ratio is 3) of 0.36 μM.
CONCLUSIONS:
The assay method demonstrates high selectivity, repeatability, stability and recovery ratio in the detection of the standard and real PA samples. We believe that the facile operation, low-cost, high sensitivity and selectivity render this CD-based "off-on" fluorescent probe an ideal sensing platform for the detection of PA.