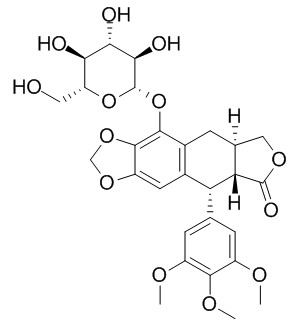
(-)-beta-Peltatin-5-O-beta-D-glucopyranoside
beta-Peltatin-5-O-beta-D-glucopyranoside provides potent cancer cell growth inhibitory activity (GI50 0.1 to <0.03 ug/mL) against a panel of six human cancer cell lines and one murine cancer cell line; it also shows modest antiproliferative activities against the A2780 ovarian cancer cell line, with the IC50 value of 4.9 ± 0.1 uM.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
PLoS One.2015, 10(5):e0127060
Phytother Res.2018, 32(12):2551-2559
Biomolecules.2024, 14(10):1257.
Indian J Pharm Sci.2022, 84(3):144-151
Mol Med Rep.2022, 26(4):299.
Applied Biological Chemistry2022, 65(12)
Molecules.2019, 24(12):E2286
Bioorg Med Chem.2020, 28(12):115553.
Chem Biol Interact.2024, 398:111103.
Evid Based Complement Alternat Med.2020, 2020:1970349.
Related and Featured Products
J Nat Prod. 2015 Jul 24;78(7):1543-7.
Antiproliferative Compounds from Cleistanthus boivinianus from the Madagascar Dry Forest.[Pubmed:
26091020 ]
METHODS AND RESULTS:
The two new lignans 3α-O-(β-D-glucopyranosyl)desoxypodophyllotoxin (1) and 4-O-(β-D-glucopyranosyl)dehydropodophyllotoxin (2) were isolated from Cleistanthus boivinianus, together with the known lignans deoxypicropodophyllotoxin (3), (±)-β-apopicropodophyllin (4), (-)-desoxypodophyllotoxin (5), (-)-yatein (6), and β-peltatin-5-O-β-D-glucopyranoside ((-)-beta-Peltatin-5-O-beta-D-glucopyranoside,7). The structures of all compounds were characterized by spectroscopic techniques.
CONCLUSIONS:
Compounds 1, 4, and 5 showed potent antiproliferative activities against the A2780 ovarian cancer cell line, with IC50 values of 33.0 ± 3.6, 63.1 ± 6.7, and 230 ± 1 nM, respectively. Compounds 2 and 7 showed only modest A2780 activities, with IC50 values of 2.1 ± 0.3 and 4.9 ± 0.1 μM, respectively, while compounds 3 and 6 had IC50 values of >10 μM. Compound 1 also had potent antiproliferative activity against the HCT-116 human colon carcinoma cell line, with an IC50 value of 20.5 nM, and compound 4 exhibited modest antiproliferative activity against the A2058 human caucasian metastatic melanoma and MES-SA human uterine sarcoma cell lines, with IC50 values of 4.6 and 4.0 μM, respectively.
J Nat Prod. 2016 Mar 25;79(3):507-18.
Antineoplastic Agents. 585. Isolation of Bridelia ferruginea Anticancer Podophyllotoxins and Synthesis of 4-Aza-podophyllotoxin Structural Modifications.[Pubmed:
26938998 ]
METHODS AND RESULTS:
Cytotoxic constituents of the terrestrial plant Bridelia ferruginea were isolated using bioactivity-guided fractionation, which revealed the presence of the previously known deoxypodophyllotoxin (1), isopicrodeoxypodophyllotoxin (2), β-peltatin (3), β-peltatin-5-O-β-D-glucopyranoside ((-)-beta-Peltatin-5-O-beta-D-glucopyranoside,3a), and the indole neoechinulin (4). As an extension of previous podophyllotoxin research, SAR studies were undertaken focused on 4-aza-podophyllotoxin structural modifications. A number of such derivatives were synthesized following modifications to the A and E rings.
CONCLUSIONS:
Such structural modifications with alkyl and 4-fluorobenzyl substituents at the 4-aza position provided the most potent cancer cell growth inhibitory activity (GI50 0.1 to <0.03 μg/mL) against a panel of six human cancer cell lines and one murine cancer cell line. Several compounds corresponding to 4'-demethylated modifications were also synthesized and found to be significantly less potent.