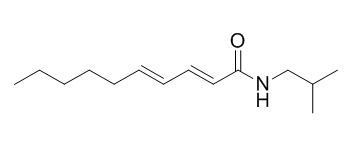
Pellitorine
Pellitorine is a potential larvicide with a specific target site and a lead molecule for the control of mosquito populations, it also shows antiprotozoal activity against Plasmodium falciparum (IC50 = 3.3 ug/mL). Pellitorine shows potent antiplatelet aggregation activity, it can suppress expression of inducible NO synthase and cyclooxygenase-2. Pellitorine also shows strong cytotoxic activities against HL60 and MCT-7 cell lines.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Mol Med Rep.2014, 9(5):1653-9
Molecules.2016, 21(6)
Biomed Pharmacother.2019, 116:108987
Molecules 2021, 26(4),1092.
Asian Pac J Cancer Prev.2021, 22(S1):97-106.
BMC Pharmacol Toxicol.2018, 19(1):5
J.Korean Soci. Food Sci. Nutri.2024, 53(11):1166-1177
Plants (Basel).2020, 9(11):1535.
J Breast Cancer.2015, 18(2):112-118
Animals (Basel).2024, 14(20):2990.
Related and Featured Products
J Agric Food Chem. 2007 Nov 14;55(23):9436-42.
Isolation and identification of antiplatelet aggregatory principles from the leaves of Piper lolot.[Pubmed:
17941696 ]
METHODS AND RESULTS:
The methanolic extract of Piper lolot, having shown potent inhibitory activity on platelet aggregation induced by arachidonic acid (AA) and platelet activating factor (PAF), was subjected to activity-guided isolation to yield twelve new amide alkaloids, piperlotine A-L (1-12), along with twenty-nine known compounds. Their structures were elucidated on the basis of spectroscopic analysis. The isolated compounds were tested for their inhibitory activity on the rabbit platelet aggregation.
CONCLUSIONS:
The compounds piperlotine A (1), piperlotine C (3), piperlotine D (4), piperlotine E (5), 3-phenyl-1-(2,4,6-trihydroxyphenyl)propan-1-one (21), 3-(4-methoxyphenyl)-1-(2,4,6-trihydroxyphenyl)propan-1-one (22), 1-trans-cinnamoylpyrrolidine (24), sarmentine (26), Pellitorine (27), methyl 3-phenylpropionate (32), and (10S)-10-hydroxypheophorbide a methyl ester (40) showed potent antiplatelet aggregation activity.
Molecules. 2014 May 20;19(5):6428-38.
Antiprotozoal activity of Achillea ptarmica (Asteraceae) and its main alkamide constituents.[Pubmed:
24853616]
METHODS AND RESULTS:
In the course of our ongoing screening of plants of the family Asteraceae for antiprotozoal activity, a CH2Cl2-extract from the flowering aerial parts of Achillea ptarmica L. (sneezewort yarrow) was found to be active in vitro against Trypanosoma brucei rhodesiense (IC50 = 0.67 µg/mL) and Plasmodium falciparum (IC50 = 6.6 μg/mL). Bioassay guided fractionation led to the isolation and identification of five alkamides from the most active fractions. Pellitorine and 8,9-Z-dehyroPellitorine are the main components of the extract. Beside these olefinic acid amides, four alkamides with diene-diyne structures were isolated. All alkamides were tested for antiprotozoal activity in vitro. Pellitorine was the most active compound so far within this study against P. falciparum (IC50 = 3.3 µg/mL), while 8,9-Z-dehydroPellitorine was most active against T. b. rhodesiense (IC50 = 2.0 µg/mL). The activity of pure Pellitorine against Plasmodium is higher than that of the crude extract and thus explains the activity of the latter.
CONCLUSIONS:
None of the isolated alkamides, however, was as active against T. b. rhodesiense as the crude extract whose antitrypanosomal activity must therfore be due to a synergistic effect of the isolated compounds or to more active yet to be identified constituents.
PLoS One. 2013 Nov 18;8(11):e80226.
Novel histopathological and molecular effects of natural compound pellitorine on larval midgut epithelium and anal gills of Aedes aegypti.[Pubmed:
24260359]
The yellow fever mosquito, Aedes aegypti, is a vector for transmitting dengue fever and yellow fever.
METHODS AND RESULTS:
In this study, we assessed the histopathological and molecular effects of Pellitorine, an isobutylamide alkaloid, on the third instar of Ae. aegypti larvae. At 5 mg/l concentration of Pellitorine, the whole body of the treated larvae became dark in color, particularly damaged thorax and abdominal regions. Pellitorine was targeted mainly on midgut epithelium and anal gills, indicating variably dramatic degenerative responses of the midgut through a sequential epithelial disorganization. The anterior and posterior midgut was entirely necrosed, bearing only gut lumen residues inside the peritrophic membranes. Pellitorine caused comprehensive damage of anal gill cells and branches of tracheole and debris was found in hemolymph of the anal gills. RT-PCR analysis indicates that the compound inhibited gene expression encoding V-type H(+)-ATPase and aquaporine 4 after treatment with 2.21 mg/l Pellitorine.
CONCLUSIONS:
These results verify that Pellitorine merits further study as a potential larvicide with a specific target site and a lead molecule for the control of mosquito populations.
Phytomedicine. 2014 Dec 15;21(14):1801-7.
Quantitative transdermal behavior of pellitorine from Anacyclus pyrethrum extract.[Pubmed:
25481393]
The plant Anacyclus pyrethrum (AP) consists of several N-alkylamides with Pellitorine as main constituent. AP extracts are known to be biologically active and some products for topical administration containing AP plant extracts are already commercially available with functional cosmeceutical claims. However, no transdermal data for Pellitorine are currently available. Therefore, our general goal was to investigate the local skin pharmacokinetics of the plant N-alkylamide Pellitorine using a Franz diffusion cell set-up.
METHODS AND RESULTS:
Two different forms were applied on human skin: purified Pellitorine and the AP extract. Our study demonstrated that Pellitorine is able to cross the stratum corneum and the subsequent skin layers. A significantly higher permeability coefficient was observed when the AP extract (Kp=2.3 × 10(-4)cm/h) was administered, compared to purified Pellitorine (Kp=1.1 × 10(-4)cm/h).
CONCLUSIONS:
With the obtained Pellitorine concentrations in the skin layers and the receptor fluid, it is concluded that local and systemic effects can be expected after topical application. Due to these findings and as a regulatory consequence, products containing reasonable concentrations of Pellitorine are recommended to be classified as a medicinal product.
Phytother Res. 2017 Apr;31(4):663-670.
Alkaloids from Piper nigrum Exhibit Antiinflammatory Activity via Activating the Nrf2/HO-1 Pathway.[Pubmed:
28185326]
METHODS AND RESULTS:
In the present study, ten alkaloids, namely chabamide (1), Pellitorine (2), retrofractamide A (3), pyrroperine (4), isopiperolein B (5), piperamide C9:1 (8E) (6), 6,7-dehydrobrachyamide B (7), 4,5-dihydropiperine (8), dehydropipernonaline (9), and piperine (10), were isolated from the fruits of Piper nigrum. Among these, chabamide (1), Pellitorine (2), retrofractamide A (3), isopiperolein B (5), and 6,7-dehydrobrachyamide B (7) exhibited significant inhibitory activity on lipopolysaccharide-induced nitric oxide (NO) production in RAW264.7 cells, with IC50 values of 6.8, 14.5, 30.2, 23.7, and 38.5 μM, respectively. Furthermore, compound 1 inhibited lipopolysaccharide-induced NO production in bone marrow-derived macrophages with IC50 value of 9.5 μM. Consistent with NO inhibition, treatment of RAW264.7 cells with chabamide (1), Pellitorine (2), and 6,7-dehydrobrachyamide B (7) suppressed expression of inducible NO synthase and cyclooxygenase-2. Chabamide (1), Pellitorine (2), and 6,7-dehydrobrachyamide B (7) induced heme-oxygenase-1 expression at the transcriptional level. In addition, compound 1 induced the nuclear translocation of nuclear factor-E2-related factor 2 (Nrf2) and upregulated the expression of Nrf2 target genes, NAD(P)H:quinone oxidoreductase 1 and γ-glutamyl cysteine synthetase catalytic subunit, in a concentration-dependent manner in RAW264.7 cells.
CONCLUSIONS:
These findings suggest that chabamide (1) from P. nigrum exert antiinflammatory effects via the activation of the Nrf2/heme-oxygenase-1 pathway; hence, it might be a promising candidate for the treatment of inflammatory diseases.
Molecules. 2010 Apr 5;15(4):2398-404.
Pellitorine, a potential anti-cancer lead compound against HL6 and MCT-7 cell lines and microbial transformation of piperine from Piper Nigrum.[Pubmed:
20428051]
METHODS AND RESULTS:
Pellitorine (1), which was isolated from the roots of Piper nigrum, showed strong cytotoxic activities against HL60 and MCT-7 cell lines. Microbial transformation of piperine (2) gave a new compound 5-[3,4-(methylenedioxy)phenyl]-pent-2-ene piperidine (3). Two other alkaloids were also found from Piper nigrum. They are (E)-1-[3',4'-(methylenedioxy)cinnamoyl]piperidine (4) and 2,4-tetradecadienoic acid isobutyl amide (5).
CONCLUSIONS:
These compounds were isolated using chromatographic methods and their structures were elucidated using MS, IR and NMR techniques.