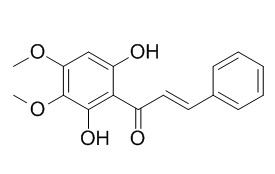
Pashanone
Pashanone possesses moderate antifungal activity, it also exhibits cytotoxic activity.
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Chengdu University of Traditional Chinese Medicine2024, 4802935.
Patanjali Research Foundation2024, ssrn.4807357
Lab Chip.2018, 18(6):971-978
ACS Synth Biol.2022, doi: 10.1021.
J. Food Composition and Anal.2022, V 109:104482.
The Korea Journal of Herbology2016, 29-35
Front Immunol.2017, 8:1542
Process Biochemistry2019, 87:213-220
BMB Rep.2020, 53(4):218-222.
Analytical Letters.2020, doi 10.1008
Related and Featured Products
Dihydropashanone
Catalog No: CFN97741
CAS No: 41997-41-5
Price: Inquiry(manager@chemfaces.com)
Pashanone
Catalog No: CFN98661
CAS No: 42438-78-8
Price: Inquiry(manager@chemfaces.com)
2',4'-Dihydroxy-3',6'-dimethoxydihydrochalcone
Catalog No: CFN98908
CAS No: 54299-52-4
Price: Inquiry(manager@chemfaces.com)
2',4'-Dihydroxy-3',6'-dimethoxychalcone
Catalog No: CFN99391
CAS No: 129724-43-2
Price: Inquiry(manager@chemfaces.com)
2'-Hydroxy-3',4',6'-trimethoxychalcone
Catalog No: CFN91489
CAS No: 91856-16-5
Price: Inquiry(manager@chemfaces.com)
2'-Hydroxy-4,3',4',6'-tetramethoxychalcone
Catalog No: CFN91409
CAS No: 91856-17-6
Price: Inquiry(manager@chemfaces.com)
Dihydropedicin
Catalog No: CFN97805
CAS No: 169234-89-3
Price: Inquiry(manager@chemfaces.com)
Pedicin
Catalog No: CFN97978
CAS No: 521-51-7
Price: Inquiry(manager@chemfaces.com)
2',6'-Dihydroxy 4'-methoxydihydrochalcone
Catalog No: CFN70346
CAS No: 35241-55-5
Price: Inquiry(manager@chemfaces.com)
Pinostrobin chalcone
Catalog No: CFN99878
CAS No: 18956-15-5
Price: $368/20mg
J Ethnopharmacol. 2011 Nov 18;138(2):633-6.
Detection of antifungal compounds in Polygonum ferrugineum Wedd. extracts by bioassay-guided fractionation. Some evidences of their mode of action.[Pubmed:
22001591]
Polygonum ferrugineum Wedd. (Polygonaceae) is used to heal infected wounds and as antiseptic, antibiotic or antifungal in the traditional Argentinean medicine. The present investigation was carried out to evaluate the antifungal properties of different extracts of aerial parts of Polygonum ferrugineum, in order to give support to its ethnopharmacological use and to isolate the compounds responsible for the antifungal properties. The most active compounds were tested for their capacity of producing hyphae malformations, similar to those previously observed for crude extracts.
METHODS AND RESULTS:
Agar Dilution Method (ADM) and Agar Overlay Bioautography (AOB) were used for bioassay-guided fractionation of the aerial part extracts against a panel of human opportunistic pathogenic fungi. The Neurospora crassa assay, followed by Optical Microscopy and Scanning Electron Microscopy observation, was used for studies of mechanisms of action.
MeOH extract and DCM and Hex sub-extracts, but not Aq, EtOAc or BuOH ones possess antifungal activity. Of the seven isolated compounds, cardamonin 2 showed a selective inhibition of Epidermophyton floccosum with a very low MIC (=6.2 μg/mL) and Pashanone 1 possessed moderate antifungal activity (MICs=25-50 μg/mL) but a broader spectrum of action. Chalcone 2, but not 1, induced swelling and shortening of the Neurospora crassa hyphae, similar as those caused by the crude DCM extract.
CONCLUSIONS:
The bioassay-guided fractionation of Polygonum ferrugineum DCM extract allowed the isolation of five active compounds. Among them, cardamonin 2 showed the highest antifungal activity and selectivity towards Epidermophyton floccosum; in addition, it induced Neurospora crassa malformations that are similar than those produced by the crude DCM extract. These results give additional support to the ethnopharmacological use of Polygonum ferrugineum as antifungal agent.
Nat Prod Res. 2011 Aug;25(14):1361-5.
A new hydrochalcone from Miliusa sinensis.[Pubmed:
21859261]
METHODS AND RESULTS:
A new dihydrochalcone 4',6'-dihydroxy-2',3',4-trimethoxydihydrochalcone (1) along with nine known compounds, Pashanone (2), dihydroPashanone (3), pinostrobin (4), 5-hydroxy-7,4'-dimethoxyflavanone (5), 5-hydroxy-6,7-dimethoxyflavanone (6), 5-hydroxy-7,8-dimethoxyflavanone (7), 24-methylencycloartane-3β,21-diol (8), liriodenine (9) and 3,5-dihydroxy-7,3',4'-trimethoxyflavone (10), were isolated from the extracts, exhibiting cytotoxic activity (n-hexane and ethyl acetate extracts) of Miliusa sinensis.
CONCLUSIONS:
The structure of (1) was elucidated by the analysis of spectral data (IR, HR-MS, EI-MS, 1D and 2D NMR).
Phytochemistry. 2006 Oct;67(19):2152-8.
An unusual homoisoflavanone and a structurally-related dihydrochalcone from Polygonum ferrugineum (Polygonaceae).[Pubmed:
16884749]
METHODS AND RESULTS:
The homoisoflavanone 5,7-dihydroxy-6-methoxy-3-(9-hydroxy-phenylmethyl)-chroman-4-one (1) and its structurally related 2',4',6'-trihydroxy-3'-methoxy-alpha-hydroxymethyl-beta-hydroxy-dihydrochalcone (2) along with the known Pashanone (3), flavokawin B (4) and cardamonin or alpinetin chalcone (5) pinostrobin (6) and 5,8-dimethoxy-7-hydroxychroman-4-one (7) were isolated from dry leaves of Polygonum ferrugineum (Polygonaceae).
CONCLUSIONS:
To our knowledge, this is the first report of the isolation of a homoisoflavanone from the Polygonum genus and the Polygonaceae family, and could be an important chemotaxonomic finding. In addition, the pattern of substitution of this homoisoflavanone is different from others previously reported.