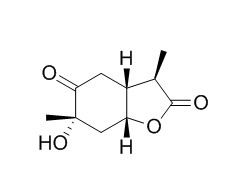
Paeonilactone A
Standard reference
Inquire / Order:
manager@chemfaces.com
Technical Inquiries:
service@chemfaces.com
Tel:
+86-27-84237783
Fax:
+86-27-84254680
Address:
1 Building, No. 83, CheCheng Rd., Wuhan Economic and Technological Development Zone, Wuhan, Hubei 430056, PRC
Providing storage is as stated on the product vial and the vial is kept tightly sealed, the product can be stored for up to
24 months(2-8C).
Wherever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20C. Generally, these will be useable for up to two weeks. Before use, and prior to opening the vial we recommend that you allow your product to equilibrate to room temperature for at least 1 hour.
Need more advice on solubility, usage and handling? Please email to: service@chemfaces.com
The packaging of the product may have turned upside down during transportation, resulting in the natural compounds adhering to the neck or cap of the vial. take the vial out of its packaging and gently shake to let the compounds fall to the bottom of the vial. for liquid products, centrifuge at 200-500 RPM to gather the liquid at the bottom of the vial. try to avoid loss or contamination during handling.
Plants2022, 11(3),294.
Int. J of Herbal Med.2023, 11(1): 06-14
Food Chem X.2024, 21:101127.
Wageningen University & Research2018, January 2018
BMC Plant Biol.2021, 21(1):60.
Sci Rep.2023, 13(1):13072.
Pharmaceuticals (Basel).2024, 17(9):1130.
Int J Mol Sci.2024, 25(17):9673.
OENO One2023, 57:3.
Biomolecules.2020, 10(2):E184
Related and Featured Products
J Org Chem. 2000 Apr 7;65(7):2122-6.
An enantioselective route to paeonilactone A via palladium- and copper-catalyzed reactions.[Pubmed:
10774035]
METHODS AND RESULTS:
We herein report on a formal total synthesis of Paeonilactone A involving palladium-, copper-, and enzyme-catalyzed reactions starting from 1,3-cyclohexadiene. The key step in the synthesis, a palladium(II)-catalyzed 1,4-oxylactonization of a conjugated diene, simultaneously introduces two of the oxygen substituents required for the target molecule. The synthesis also includes our recently developed copper(I)-catalyzed cross-coupling reaction between dienyltriflates with Grignard reagents, introducing one of the methyl groups present in the target molecule.
CONCLUSIONS:
This new approach toward Paeonilactone A allows complete control of all four stereogenic centers and is the first enantioselective route toward Paeonilactone A starting from an achiral substrate.
Fitoterapia. 2007 Jan;78(1):76-8.
Monoterpene glycosides from Paeonia delavayi.[Pubmed:
17067761]
METHODS AND RESULTS:
A new monoterpene glycoside, 4-O-methyl-4''-hydroxy-3''-methoxy-paeoniflorin (1), was isolated from the root cortex of Paeonia delavayi along with the known paeoniflorin, oxypaeoniflorin, benzoylpaeoniflorin, benzoyloxypaeoniflorin, albiflorin and a Paeonilactone A.